Variability in the anti-tumor effect of tegafur-uracil depending on histologic types of lung cancer
Introduction
To date, lung cancer (LC) has been considered a leading cause of cancer-related death worldwide; this is also the case with Korea (1-3). It is diagnosed at inoperable locally advanced or metastatic stage. In addition, it may frequently show distant metastasis even in the early stage of initial diagnosis after tumor resection (4). This indicates that most of the patients with LC are indicated in systemic chemotherapy. LC is classified by two histopathologic subtypes: non-small cell lung cancer (NSCLC) and small cell lung cancer (SCLC). The main subtypes of NSCLC are adenocarcinoma, squamous cell (Sq) carcinoma and large cell carcinoma (5). The histologic subtypes of LC is a key factor that determines the selection of optimal chemotherapy regimens. For instance, pemetrexed inhibits multiple enzymes affecting purine and pyrimidine synthesis. The primary target of pemetrexed is thymidylate synthase (TS), which is a key enzyme in de novo DNA synthesis. TS, a key enzyme for thymidine nucleotide biosynthesis is an obvious target for cytotoxic agents since thymidine is the only nucleotide precursor specific to DNA. High TS expression is associated with poor clinical outcomes, because pemetrexed cannot fully inhibit elevated TS activity. TS expression is higher in SCLC than NSCLC, and higher in Sq NSCLC than non-Sq NSCLC, so pemetrexed is more effective for the treatment of non-Sq NSCLC (6-8).
Tegafur-uracil (UFT) is a combination of two drugs, tegafur and uracil. Tegafur is a pro-drug of 5-fluorouracil (5-FU) which is activated and kills tumor cells mainly through inhibition of TS, and uracil is an inhibitor of dihydropyrimidine dehydrogenase (DPD) involved in the degradation of 5-FU. Therefore, the co-administration of tegafur with uracil produces a constant reserve of 5-FU concentration in tumor cell (9,10). Because TS is target of UFT in common with pemetrexed, we thought there might be variability in the clinical efficacy of UFT depending on histological types of LC (9-12).
Several studies have shown that UFT is an effective postoperative adjuvant therapy regimen (13,14). It has also been shown, however, that its anti-tumor effect remains minimal in patients with advanced LC (15). Still, however, there is a paucity of data regarding whether the anti-tumor efficacy of UFT varies depending on histological subtypes of LC. In this study, we examined the variability of the anti-tumor efficacy of UFT monotherapy depending on histological subtypes of LC.
Patients and methods
Study population
We retrospectively reviewed the clinical records of the patients with LC who were treated with UFT across all treatment lines at the Chonnam National University Hwasun Hospital in Korea between January 2008 and July 2013. Inclusion criteria for the current study are as follows: (I) the patients aged between 19 and 80 years; (II) the patients with ≥1 measurable disease according to the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1; (III) the patients with Eastern Cooperative Oncology Group (ECOG) performance status (PS) of ≤3; (IV) The patients with a life expectancy of ≥12 weeks; (V) the patients with adequate bone marrow, renal and hepatic function. Exclusion criteria for the current study are as follows: (I) the patients with severe bacterial infection; (II) the LC patients with who have previous cancer or synchronous cancer other than basal cell skin cancer or carcinoma in situ of cervix; (III) women with child-bearing potential; (IV) women who are pregnant or breast-feeding. Clinicopathologic and follow-up data were retrieved from medical records through March 10, 2014. All the patients had histologically-proven LC, who were divided into three groups: the Sq NSCLC group, the non-Sq NSCLC group and the SCLC group.
The current study was approved by the Institutional Review Board (IRB) of Chonnam National University Hwasun Hospital (IRB approval number: CNUHH-2014-097). Informed consent was waived due to the retrospective nature of the current study.
Treatment
UFT was orally administered at a dose of tegafur of 200-1,200 mg/day in divided doses, for which attending physicians determined the dosage based on the age, general condition and body surface area of the patients. Treatment was continued until the patients showed signs of disease progression or unacceptable toxicity or until they or attending physicians decided on treatment discontinuation.
Evaluation
The tumor response to chemotherapy was evaluated on computed tomography (CT) scans at a 2-month interval. If applicable, however, it was also evaluated whenever there were signs of tumor progression. The objective tumor response was evaluated based on the RECIST version 1.1 (16). A complete response (CR) was defined as the disappearance of all clinical and radiologic evidence of tumor; a partial response (PR) was defined as a decrease of 30% or more in the sum of longest diameters of all target measurable lesions; and a progressive disease (PD) was defined as an increase of more than 20% of the sum of longest diameters of all target measurable lesions or the appearance of new lesions. All other circumstances were considered to indicate stable disease (SD). The overall response rate (ORR) was defined as the sum of best tumor response of CR and PR. The disease control rate (DCR) was defined as the sum of best tumor response of CR, PR, and SD.
The overall survival (OS) was calculated from the start date of the treatment until death or until the last follow-up visit, for which we considered all deaths. Progression-free survival (PFS) included the time from the first cycle of chemotherapy to documented progression until death from any cause or until the date of the last follow-up visit in survivors from LC. Toxicities were regularly evaluated throughout the treatment period based on the National Cancer Institute Common Toxicity Criteria (NCICTC) version 4.0 (17).
Statistical analyses
We compared baseline and clinical characteristics of the patients between the three groups using the Chi-square test. In addition, we also compared continuous variables using one-way analysis of variance (ANOVA). We plotted the Kaplan-Meier survival curve and compared the OS and PFS between the three groups using the log-rank test. Furthermore, we compared the objective tumor response between the three groups using the Chi-square test. Statistical analysis was done using the SPSS version 18 (SPSS Inc, Chicago, IL), for which a P value of <0.05 was considered statistically significant.
Results
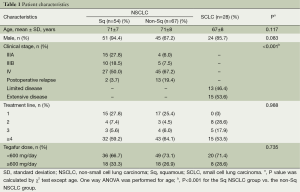
In the current study, we enrolled a total of 149 patients (n=149), who were assigned to the Sq NSCLC group (n=54), the non-Sq NSCLC group (n=67) and the SCLC group (n=28). Baseline and clinical characteristics of the patients are represented in Table 1. There were no significant differences in baseline and clinical characteristics, except for the clinical stage of LC, between the three groups.
Full table
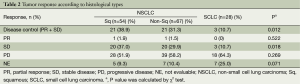
There was no CR in our series. ORR was 1% in the Sq NSCLC group and the non-Sq NSCLC group and 0% in the SCLC group (P=0.522) (Table 2). DCR was higher in the Sq NSCLC group and the non-Sq NSCLC group as compared with the SCLC group (38.9% vs. 31.3% vs. 10.7%; P=0.012). But there was no significant difference in the DCR between the Sq NSCLC group and the non-Sq NSCLC group (P=0.444). A waterfall plot of the best response of target lesions is shown in Figure 1. There was a tumor shrinkage in 11% (6/54) of the Sq NSCLC group and 9% (6/67) of the non-Sq NSCLC group. In addition, there was one patient who achieved a PR. In patients with a tumor shrinkage that did not meet PR criteria, average tumor shrinkage was 12.6% (range, 3-26%) in Sq NSCLC and 9.0% (range, 3-19%) in non-Sq NSCLC. But there was no tumor shrinkage in the SCLC group. There was no significant difference in DCR according to line of therapy in Sq (P=0.628) and non-Sq NSCLC (P=0.135).
Full table
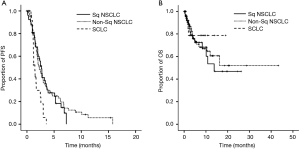
On the Kaplan-Meier curve, the median PFS was 2.68 months in the Sq NSCLC group, 2.25 months in the non-Sq NSCLC group and 1.46 months in the SCLC group; these results indicate that it was significantly higher in the NSCLC group as compared with the SCLC group (P=0.004 for three groups and P=0.773 for two groups except for the SCLC group at the log-rank test, Figure 2A). The median OS was 13.79 months in the Sq NSCLC group, but it showed no significant difference between the non-Sq NSCLC group and the SCLC group (P=0.795 for three groups and P=0.745 for two groups except for the SCLC group at the log-rank test (Figure 2B).
To examine the difference in the efficacy depending on the dose of UFT, we compared the tumor response, OS and PFS between the two UFT regimens (the dose of UFT <600 or ≥600 mg/day). This showed that the DCR was lower in the group of the dose of UFT <600 mg/day as compared with that of ≥600 mg/day (43.2% vs. 24.8%, P=0.032). But there were no significant differences in the ORR, PFS and OS between the two regimens.
In our series, there were eight patients who experienced serious adverse events (SAEs). Grade 3 hematologic toxicities include one case of anemia, one case of thrombocytopenia and one case of leucopenia. In addition, grade 3 non-hematologic toxicities include two cases of anorexia, one case of diarrhea, one case of fatigue and one case of pneumonia. Moreover, grade 4 hematologic toxicities include one case of neutropenia accompanied by grade 3 leucopenia. Furthermore, grade 4 non-hematologic toxicities include one case of pneumonia.
Discussion
UFT is a 5-FU derivative chemotherapeutic agent; it was developed and has been widely used in Japan, whose indications include solid tumors such as malignant tumors of the gastrointestinal tract, lung or breast (10). According to previous prospective trials and a meta-analysis, UFT monotherapy was an effective postoperative adjuvant chemotherapy regimen in patients with early NSCLC (13,14,18). A phase II clinical study showed, however, that it had a minimal anti-tumor effect in patients with advanced NSCLC (15). As compared with previous published studies in this series (n=21), we enrolled a larger number of patients (n=121) with advanced NSCLC who had been treated with UFT monotherapy. But our results showed a lower response rate as compared with previous reports (1.7% vs. 6.3%) (15). Presumably, this might be because we enrolled more than 60% of the patients who were given UFT as ≥ the 4th line of treatment. This indicates, however, that UFT might be another treatment option in heavily treated patients because it showed a lower toxicity despite a minimal anti-tumor effect.
We enrolled both the patients with NSCLC and those with SCLC. To date, however, few studies have examined the variability in the efficacy of UFT monotherapy depending on histological types of LC including SCLC and NSCLC. Both experimental and clinical studies have shown that the resistance to 5-FU is associated with the high activity of DPD, mediating the rapid degradation of 5-FU, and that of TS, showing a resistance to 5-FU, in patients with LC (11,12,19,20). The degree of TS expression was higher in patients with SCLC as compared with NSCLC and in those with Sq NSCLC as compared with those with non-Sq NSCLC. It has been reported that there was an inverse correlation between the degree of TS expression and that of tumor response in patients with NSCLC who had been treated with pemetrexed, an anti-folate drug mainly involved in the inhibition of TS activity, because pemetrexed cannot fully inhibit elevated TS activity (21). It can therefore be inferred that there is an inverse correlation between the degree of TS expression and that of tumor response following UFT monotherapy in patients with non-Sq NSCLC as compared with those with other histological types including Sq NSCLC and SCLC because UFT kills tumor cells mainly through inhibition of TS similar to that of pemetrexed. Our results showed that the DCR and PFS were significantly higher in patients with NSCLC as compared with those with SCLC. But there were no significant differences in them between the Sq NSCLC group and the non-Sq NSCLC group. Presumably, this might be because there would be no significant difference in the degree of the anti-tumor effect of UFT between the patients with non-Sq NSCLC as compared with those with Sq NSCLC because that of TS expression is lower and that of DPD expression is higher in the patients with non-Sq NSCLC as compared with those with Sq NSCLC (9).
There are several limitations of the current study shown as below: (I) the current study was conducted in a single-institution retrospective setting; (II) we failed to analyze some factors, such as PS, because there were some missing data; (III) we determined the dose of UFT and the timing of response evaluation solely based on our subject judgment. Therefore, this might have caused a bias; (IV) because UFT is not approved by the U.S. Food and Drug Administration, it would be of little interest to U.S. oncologist. However, UFT has been approved in many countries of Western Europe, Latin America, Asia, and South Africa, so it would be of interest to oncologists in these countries; (V) we could not investigate the direct relationship between TS expression level and the efficacy of UFT because we don’t have the data of TS expression in tumor specimens from LC patients.
In conclusion, our results indicate that the degree of the anti-tumor effect of UFT was higher in patients with NSCLC as compared with SCLC. But it showed no significant difference between the patients with Sq NSCLC and those with non-Sq NSCLC.
Acknowledgements
Funding: This study was supported by a grant (HCRI 14017-1) Chonnam National University Hwasun Hospital Institute for Biomedical Science.
Disclosure: The authors declare no conflict of interest.
References
- Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011;61:69-90. [PubMed]
- Jung KW, Won YJ, Kong HJ, et al. Cancer statistics in Korea: incidence, mortality, survival and prevalence in 2010. Cancer Res Treat 2013;45:1-14. [PubMed]
- In KH, Kwon YS, Oh IJ, et al. Lung cancer patients who are asymptomatic at diagnosis show favorable prognosis: a korean Lung Cancer Registry Study. Lung Cancer 2009;64:232-7. [PubMed]
- Spira A, Ettinger DS. Multidisciplinary management of lung cancer. N Engl J Med 2004;350:379-92. [PubMed]
- Travis WD, Brambilla E, Noguchi M, et al. International association for the study of lung cancer/american thoracic society/european respiratory society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol 2011;6:244-85. [PubMed]
- Adjei AA. Pharmacology and mechanism of action of pemetrexed. Clin Lung Cancer 2004;5 Suppl 2:S51-5. [PubMed]
- Joerger M, Omlin A, Cerny T, et al. The role of pemetrexed in advanced non small-cell lung cancer: special focus on pharmacology and mechanism of action. Curr Drug Targets 2010;11:37-47. [PubMed]
- Scagliotti G, Brodowicz T, Shepherd FA, et al. Treatment-by-histology interaction analyses in three phase III trials show superiority of pemetrexed in nonsquamous non-small cell lung cancer. J Thorac Oncol 2011;6:64-70. [PubMed]
- Tanaka F, Wada H, Fukushima M. UFT and S-1 for treatment of primary lung cancer. Gen Thorac Cardiovasc Surg 2010;58:3-13. [PubMed]
- Malet-Martino M, Martino R. Clinical studies of three oral prodrugs of 5-fluorouracil (capecitabine, UFT, S-1): a review. Oncologist 2002;7:288-323. [PubMed]
- Nakano J, Huang C, Liu D, et al. Evaluations of biomarkers associated with 5-FU sensitivity for non-small-cell lung cancer patients postoperatively treated with UFT. Br J Cancer 2006;95:607-15. [PubMed]
- Miyoshi T, Kondo K, Toba H, et al. Predictive value of thymidylate synthase and dihydropyrimidine dehydrogenase expression in tumor tissue, regarding the efficacy of postoperatively administered UFT (tegafur+uracil) in patients with non-small cell lung cancer. Anticancer Res 2007;27:2641-8. [PubMed]
- Kato H, Ichinose Y, Ohta M, et al. A randomized trial of adjuvant chemotherapy with uracil-tegafur for adenocarcinoma of the lung. N Engl J Med 2004;350:1713-21. [PubMed]
- Wada H, Hitomi S, Teramatsu T. Adjuvant chemotherapy after complete resection in non-small-cell lung cancer. West Japan Study Group for Lung Cancer Surgery. J Clin Oncol 1996;14:1048-54. [PubMed]
- Keicho N, Saijo N, Shinkai T, et al. Phase II study of UFT in patients with advanced non-small cell lung cancer. Jpn J Clin Oncol 1986;16:143-6. [PubMed]
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-47. [PubMed]
- National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE). Version 4.0. Available online: http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf
- Hamada C, Tanaka F, Ohta M, et al. Meta-analysis of postoperative adjuvant chemotherapy with tegafur-uracil in non-small-cell lung cancer. J Clin Oncol 2005;23:4999-5006. [PubMed]
- Oguri T, Achiwa H, Bessho Y, et al. The role of thymidylate synthase and dihydropyrimidine dehydrogenase in resistance to 5-fluorouracil in human lung cancer cells. Lung Cancer 2005;49:345-51. [PubMed]
- Huang CL, Yokomise H, Kobayashi S, et al. Intratumoral expression of thymidylate synthase and dihydropyrimidine dehydrogenase in non-small cell lung cancer patients treated with 5-FU-based chemotherapy. Int J Oncol 2000;17:47-54. [PubMed]
- Wang L, Wang R, Pan Y, et al. The pemetrexed-containing treatments in the non-small cell lung cancer is -/low thymidylate synthase expression better than +/high thymidylate synthase expression: a meta-analysis. BMC Cancer 2014;14:205. [PubMed]



