Clinicopathological and prognostic significance of programmed cell death ligand1 (PD-L1) expression in patients with non-small cell lung cancer: a meta-analysis
Introduction
Non-small cell lung cancer (NSCLC) is one of the leading causes of cancer-related deaths worldwide (1). Although recent advances in multidisciplinary therapies have improved treatment outcomes, the overall prognosis for NSCLC remains poor. Novel therapeutic strategies, including immunotherapy, are underwent investigation.
Programmed cell death 1 (PD-1) is a key immune checkpoint protein expressed on activated T cells and plays a crucial role in tumor immune escape (2-4). The ligand of PD-1, programmed cell death ligand 1 (PD-L1), which is upregulated in various types of cancers has been identified delivering negative costimulatory signals and suppressing lymphocyte function within the tumor microenvironment by engaging its receptor (5-7). Blocking the PD-1/PD-L1 pathway can enhance the anti-tumor activity of cytotoxic T cell in vitro (8,9). Furthermore, anti-PD-1 (10) and anti-PD-L1 (11) monoclonal antibodies have shown promising clinical activity in several malignancies, including NSCLC.
In previous phase I clinical trials, patients with NSCLC have shown durable and significant response to anti-PD-1 and anti-PD-L1 antibody. Studies also suggested that PD-L1 protein expression on cancer cells may predict favorable response to PD-1/PD-L1 directed therapy (7,10,12,13). However, there are finite and conflicting data on the prevalence and the prognostic role of PD-L1 expression in NSCLC. Whether discrepancy in these results is attributed to limited sample size or genuine heterogeneity is still confusing. A meta-analysis was carried out to evaluate the clinicopathological and prognostic significance of PD-L1 expression in patients with NSCLC.
Materials and methods
Search strategy
A comprehensive literature search of electronic databases PubMed, Embase, Web of science and China National Knowledge Infrastructure (CNKI) was performed up to July 10, 2014. Studies were selected using the following search terms: “lung” and “cancer or neoplasm or carcinoma” and “PD-L1 or programmed cell death ligand 1”. All abstracts from the American Society of Clinical Oncology (ASCO) conferences held between January 2000 and June 2014 were also searched for relevant researches. The eligible reports were identified by two reviewers (Zhen-Kui Pan and Feng Ye), and controversial studies were adjudicated by a third reviewer (Jing-Xun Wu).
Selection criteria
We collected all eligible articles about relationship between PD-L1 expression and clinicopathological features or clinic outcome of NSCLC in this meta-analysis. Studies meeting the following inclusion criteria were included: (I) PD-L1 protein expression evaluated in the primary NSCLC tissues; (II) research that revealed the relationship between PD-L1 expression and clinicopathological parameters or prognosis of NSCLC; (III) studies regarding the prognosis provided sufficient information to estimate hazard ratio (HR) about overall survival (OS) and 95% confidence interval (CI) ; (IV) if there were multiple articles based on similar populations, only the largest or the most recent article was included. The exclusion criteria included the following: (I) letters, reviews, case reports, conference abstracts, editorials and expert opinion; (II) patients had received previous chemotherapy or radiotherapy.
Data extraction
Two investigators (Feng Ye and Xuan Wu) independently extracted data from eligible studies. Disagreements were resolved by discussion and consensus. Two investigators reviewed all of researches that met inclusion and exclusion criteria. The following information was recorded for each study: name of the first author, year of publication, sample source, number of cases, detection methods, clinicopathological parameters, tumor node metastasis (TNM) stage, definition of PD-L1 positive, PD-L1 positive expression and patient survival. If the HR or standard errors (SE) were not reported in included studies, we calculate or estimate the HR from available data or Kaplan-Meier curves using the methods reported by Tierney et al. (14).
Assessment of study quality
Two authors (Zhen-Kui Pan and Jing-Xun Wu) independently assessed the quality of all studies on the basis of a 9-scores system of the Newcastle-Ottawa Scale (NOS) (15). Discrepancies in the score were resolved through discussion between the authors. Each study included in the meta-analysis was judged on three broad perspectives: (I) the selection of the groups of study (four items, one score each); (II) the comparability (one item, up to two scores); (III) the ascertainment of either the exposure or outcome of interest (three items, one score each). A score presents a high quality choice of individual study.
Statistical analysis
Analysis was performed using the Stata 12.0 (Stata Corporation, Texas, US) and Review Manager 5.2 (Cochrane Collaboration, Oxford, UK). Comparisons of dichotomous measures were performed by pooled estimates of odds ratios (ORs), as well as their 95% CI. A P<0.05 was considered as statistical significance. Heterogeneity was tested using the Chi-square test with significance being set at P<0.10, the total variation among studies was estimated by I square. If there was heterogeneity among studies, we used a random effect model to pool the ORs; otherwise, a fixed effect model was selected. The potential for publication bias was assessed using the Begg rank correlation method and the Egger weighted regression method.
Results
Search results
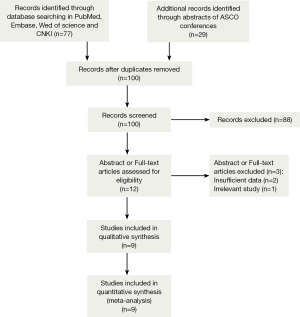
A total of 1,550 records were identified by the primary computerized literature search. After screening the titles and abstracts, 12 articles were further reviewed in detail. As indicated in the search flow diagram (Figure 1), nine studies (16-24) were finally included in the meta-analysis.
Study characteristics
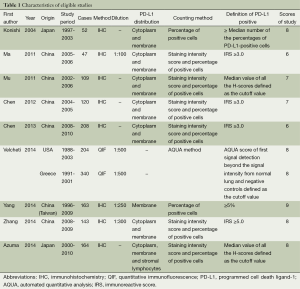
Nine studies published from 2004 to 2014 were eligible for meta-analysis. Their characteristics were summarized in Table 1. A total of 1550 patients from China, Japan, Greece and United States were enrolled. The study carried out by Velcheti et al. enrolled two cohorts of patients, including 340 cases from Greece and 204 cases from USA (21). PD-L1 protein expression was evaluated by the method of immunohistochemistry (IHC) and quantitative immunofluorescence (QIF). The definition of positive expression of PD-L1 varied among the studies. The expression of PD-L1 was found in 660 participants (42.6%).
Full table
Qualitative assessment
The study quality was assessed using the Newcastle-Ottawa quality assessment scale, generating scores ranging from 6 to 9 (with a mean of 7.4), with a higher value indicating better methodology. The results of quality assessment are also shown in Table 1.
Quantitative synthesis
PD-L1 expression and clinicopathological features
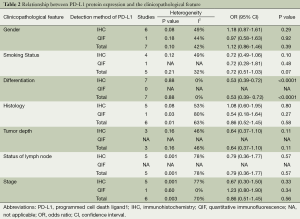
Seven out of nine studies evaluated the correlation of PD-L1 expression with gender. It is found that PD-L1 expression was not different in male and female with NSCLC. (male vs. female, OR =1.12; 95% CI: 0.86-1.46; P=0.39; Table 2).
Full table
Five studies examined the relation between PD-L1 expression and smoking status. The combined OR suggested there was no relation between these two factors (smokers vs. non-smokers, OR =0.72; 95% CI: 0.51-1.03; P=0.07; Table 2).
The association between PD-L1 expression and histological type of NSCLC was investigated in six studies. The outcomes were heterogeneous (P=0.01; I2=63%). Therefore, a random-effect model was used for the meta-analysis. PD-L1 expression was not significantly different between adenocarcinoma (AC) and squamous cell carcinoma (SCC) (AC vs. SCC, OR =0.86; CI: 0.52-1.45; P=0.58; Table 2).
In seven studies, the association of PD-L1 expression with tumor differentiation of NSCLC was investigated. The combined OR revealed positive PD-L1 expression was significantly related to poor tumor differentiation (well/moderate vs. poor differentiation, OR =0.53; 95% CI: 0.39-0.72; P<0.0001; Table 2).
Three, five and six out of eight studies demonstrated the relationship of PD-L1 expression to invasive depth of tumor, status of lymph node metastasis and TNM stage respectively. The result of meta-analysis showed PD-L1 expression was not associated with tumor size (OR =0.64; 95% CI: 0.37-1.10; P=0.11; Table 2), status of lymph node (OR =0.79; 95% CI: 0.36-1.77; P=0.57; Table 2) and TNM stage (OR =0.86; 95% CI: 0.51-1.45; P=0.56; Table 2). The heterogeneity was observed in the analysis of PD-L1 expression with status of lymph node (P=0.001; I2=78%) and TNM stage (P=0.003; I2=70%), so a random-effect model was used.
Furthermore, we stratified the extracted data by the detection methods of PD-L1 expression: the result showed PD-L1 expression was not associated with most clinicopathological characteristics (gender, smoking status, histological type, invasive depth of tumor, status of lymph node metastasis and TNM stage), regardless of the detection methods (Table 2).
PD-L1 as a prognostic factor for NSCLC
Seven out of the nine studies included have estimated the relationship between OS and PD-L1 expression. The pooled HR for OS showed expression of PD-L1 was adversely associated with the survival outcome of NSCLC (positive vs. negative PD-L1 expression, HR =1.47; 95% CI: 1.19-1.83; P=0.0004; Figure 2). Heterogeneity among the studies was not statistically significant (P=0.09; I2=45%).
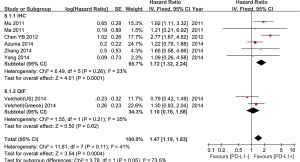
In subgroup analysis, the stratified group based on different detection methods revealed positive expression of PD-L1 by IHC was related to worse prognosis (HR =1.72; 95% CI: 1.32-2.24), but PD-L1 positivity by QIF alone could not show any predictive value (Figure 2).
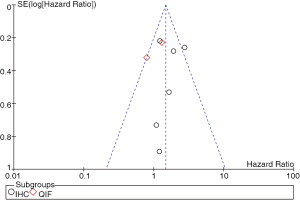
Publication bias
Heterogeneity testing and publication bias analyses were performed among the studies based on detecting methods of PD-L1 expression. Egger’s and Begg’s test indicated no publication bias among these studies regarding HR about OS with P values of 0.764 and 0.657 respectively. The funnel plots were largely symmetric (Figure 3).
Discussion
Though the using of modern therapeutic strategies have led to improved treatment outcomes, NSCLC has a 5-year OS rate of just 16% for all stage (25) and novel approaches of treatment are needed.
Advances in exploring the role of immune system in tumor immunosurveillance have resulted in the recognition that tumors can evade immune attack via the dysregulation of co-inhibitory or checkpoint signals. This has led to the development of a series of immunotherapeutic agents targeting the immune checkpoint pathway (26). Emerging data has demonstrated promising outcome of immunotherapy by anti-PD-1 and anti-PD-L1 antibody in NSCLC.
Several clinical trials of anti-PD-1 and anti-PD-L1 monoclonal antibody, including Nivolumab (BMS-936558, MDX-1106, ONO-4538) (10,27), Lambrolizumab (MK-3475) (13), MPDL3280A (12), BMS-936559 (11), and MEDI4736 (28), have reported durable clinical activity in NSCLC with tolerable adverse events. Besides, the studies also revealed that expression of PD-L1 protein in tumor cell may predict the treatment outcome (7,10,12,13). Investigating the association of PD-L1 protein expression with clinicopathoological parameters and prognosis will help the further research on PD-1/PD-L1 pathway inhibitor in NSCLC.
The correlation between PD-L1 expression and NSCLC has been studied by many studies. However, the results were inconsistent and conflicting. For procuring a reasonable conclusion, we combined 9 eligible studies including 1,550 cases to perform this meta-analysis.
In published data, nearly all the clinicopathological features, including histological type, differentiation, smoking status, tumor depth, status of lymph node metastasis and TNM stage, have been reported associated with PD-L1 protein expression in NSCLC. But most of them were not strongly confirmed by other studies. Only one study (20) showed PD-L1 expression was correlated with smoking status. With regards to histology, positive results were reported in two studies. PD-L1 expression was found to be associated with squamous cell carcinoma by Velcheti et al. (21), whilst in contrast with adenocarcinoma in another study by Mu et al. (18). Besides, tumor depth, lymph node status and TNM stage were founded related to PD-L1 expression in one (23), three (17,20,23) and four (17,19,21,23) studies, respectively. However, when we pooled the data together, none of the clinicopathological features mentioned above (histological type, smoking status, tumor depth, lymph node status and TNM stage) was associated with PD-L1 expression. Though there were just two studies showed PD-L1 expression were correlated with tumor differentiation (19,22), our meta-analysis suggested tumor differentiation is the unique clinicopathological parameter associated with PD-L1 expression in NSCLC. The discordances among previous and current analysis may result from inadequate sample size, heterogeneous study population and variable definitions of PD-L1 expression.
In six previous studies with 1,127 NSCLCs (17-19,21,23,24), PD-L1 expression was considered a prognostic factor for OS. The result of five studies (17-19,23,24) indicated PD-L1 expression is a risk factor for poor OS, which was different from the remaining one (21), in which 544 patients from two cohorts were included. Although the ultimate effects of PD-L1 signaling in the tumor are not completely understood, it is commonly believed that PD-L1 expression in tumor cell would weaken their immunogenicity, inhibit immune response cells, induce apoptosis of tumor antigen-specific T lymphocytes, decrease TCR mediated proliferation and cytokine production, down regulate the function of T cells, and lead to immune escape and tumor progression (5,29-34). On the other hand, some researchers postulated that PD-L1 expression highlights the existence of antitumor responses as well. Even though the resistance mechanisms will finally allow tumor cells to survive, tumor growth may still be partially inhibited and lead to better survival than patients with little antitumor responses (35). Whereas the conflicting data were presented by former studies, the pooled HR showed a remarkable negative correlation between PD-L1 expression and survival in our meta-analysis.
Stratified data based on the detection methods of PD-L1 expression were analysised in our research. The relationships of PD-L1 expression and clinicopathological characteristics (gender, smoking status, histological type, invasive depth of tumor, status of lymph node metastasis and TNM stage) were not different between two subgroups. However, analysis suggested the prognosis was only associated with positive expression of PD-L1 by IHC, but not with that by QIF. IHC and QIF were both antibody-based detection methods widely used in conventional researches of protien exprssion, but the definition of PD-L1 positivity by these two methods were different. Researches by IHC defined positive PD-L1 expression by staining intensity score and percentage of positive cells. Whereas, positive PD-L1 expression by QIF was defined by signal intensity. Discordant results may attributed to different definition of positive PD-L1. Besides, diverse antibody used in the studies may also lead to inconsistent results.
Efforts were made to conduct a comprehensive analysis, but some limitations still should be acknowledged. First, the detecting and evaluating method of PD-L1 protein expression was not well defined. PD-L1 positivity was judged by different antibodies and assays according to various criteria. Second, the survival analysis was not performed by multivariate analyses in most studies reported; we calculated or estimated some of the HRs from available data or Kaplan-Meier curves. Third, six out of eight included studies were hold in china and only one included study was carried out in the western country. The results need to be confirmed in a wider range of population. Fourth, the number of studies included is relatively small.
Conclusions
Our meta-analysis indicated PD-L1 protein expression was not associated with common clinicopathological characteristics of NSCLC, including gender, smoking status, histological type, invasive depth of tumor, status of lymph node metastasis and TNM stage, except for tumor differentiation. It was a poor prognostic biomarker for NSCLC. Further large-scale clinical studies should be performed to investigate the precise clinicopathological and prognostic significance of PD-L1 in NSCLC under uniform testing standard.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin 2012;62:10-29. [PubMed]
- Keir ME, Butte MJ, Freeman GJ, et al. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol 2008;26:677-704. [PubMed]
- Nishimura H, Honjo T. PD-1: an inhibitory immunoreceptor involved in peripheral tolerance. Trends Immunol 2001;22:265-8. [PubMed]
- Watanabe N, Gavrieli M, Sedy JR, et al. BTLA is a lymphocyte inhibitory receptor with similarities to CTLA-4 and PD-1. Nat Immunol 2003;4:670-9. [PubMed]
- Dong H, Strome SE, Salomao DR, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med 2002;8:793-800. [PubMed]
- Hansen JD, Du Pasquier L, Lefranc MP, et al. The B7 family of immunoregulatory receptors: a comparative and evolutionary perspective. Mol Immunol 2009;46:457-72. [PubMed]
- Sznol M, Chen L. Antagonist antibodies to PD-1 and B7-H1 (PD-L1) in the treatment of advanced human cancer. Clin Cancer Res 2013;19:1021-34. [PubMed]
- Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature 2011;480:480-9. [PubMed]
- Weber J. Immune checkpoint proteins: a new therapeutic paradigm for cancer--preclinical background: CTLA-4 and PD-1 blockade. Semin Oncol 2010;37:430-9. [PubMed]
- Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012;366:2443-54. [PubMed]
- Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med 2012;366:2455-65. [PubMed]
- Soria JC. Clinical activity, safety and biomarkers of PD-L1 blockade in non-small cell lung cancer (NSCLC): additional analyses from a clinical study of the engineered antibody MPDL3280A (anti-PDL1). European Cancer Congress 2013;Abstract 3408.
- Garon E, Balmanoukian A, Hamid O, et al. Preliminary clinical safety and activity of MK-3475 mono-therapy for the treatment of previously treated patients with non-small cell lung cancer. IASLC 15th World Conference on Lung Cancer 2013;Abstract MO18.02.
- Tierney JF, Stewart LA, Ghersi D, et al. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007;8:16. [PubMed]
- Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
- Konishi J, Yamazaki K, Azuma M, et al. B7-H1 expression on non-small cell lung cancer cells and its relationship with tumor-infiltrating lymphocytes and their PD-1 expression. Clin Cancer Res 2004;10:5094-100. [PubMed]
- Ma W, Luo D, Chen Y, et al. Expression and clinical significance of PD-L1 and PD-1 in non-small cell lung cancer. The Journal of Practical Medicine (Chinese) 2011;27:1551-54.
- Mu CY, Huang JA, Chen Y, et al. High expression of PD-L1 in lung cancer may contribute to poor prognosis and tumor cells immune escape through suppressing tumor infiltrating dendritic cells maturation. Med Oncol 2011;28:682-8. [PubMed]
- Chen YB, Mu CY, Huang JA. Clinical significance of programmed death-1 ligand-1 expression in patients with non-small cell lung cancer: a 5-year-follow-up study. Tumori 2012;98:751-5. [PubMed]
- Chen YY, Wang LB, Zhu HL, et al. Relationship between programmed death-ligand 1 and clinicopathological characteristics in non-small cell lung cancer patients. Chin Med Sci J 2013;28:147-51. [PubMed]
- Velcheti V, Schalper KA, Carvajal DE, et al. Programmed death ligand-1 expression in non-small cell lung cancer. Lab Invest 2014;94:107-16. [PubMed]
- Yang CY, Lin MW, Chang YL, et al. Programmed cell death-ligand 1 expression in surgically resected stage I pulmonary adenocarcinoma and its correlation with driver mutations and clinical outcomes. Eur J Cancer 2014;50:1361-9. [PubMed]
- Zhang Y, Wang L, Li Y, et al. Protein expression of programmed death 1 ligand 1 and ligand 2 independently predict poor prognosis in surgically resected lung adenocarcinoma. Onco Targets Ther 2014;7:567-73. [PubMed]
- Azuma K, Ota K, Kawahara A, et al. Association of PD-L1 overexpression with activating EGFR mutations in surgically resected nonsmall-cell lung cancer. Ann Oncol 2014;25:1935-40. [PubMed]
- American Cancer Society. Cancer Facts & Figures 2012. Available online: http://www.cancer.org/acs/groups/content/@epidemiologysurveilance/documents/document/acspc-033423.pdf
- Sundar R, Soong R, Cho BC, et al. Immunotherapy in the treatment of non-small cell lung cancer. Lung Cancer 2014;85:101-9. [PubMed]
- Brahmer JR, Horn L, Antonia SJ, et al. Survival and long-term follow-up of the phase I trial of nivolumab (Anti-PD-1; BMS-936558; ONO-4538) in patients (pts) with previously treated advanced non-small cell lung cancer (NSCLC). ASCO Annual Meeting 2013;Abstract 8030.
- Khleif S, Lutzky J, Segal N, et al. MEDI4736, an anti-PD-L1 antibody with modified Fc domain: preclinical evaluation and early clinical results from a phase 1 study in patients with advanced solid tumors. European Cancer Congress 2013;Abstract 802.
- Iwai Y, Ishida M, Tanaka Y, et al. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci U S A 2002;99:12293-7. [PubMed]
- Tamura H, Ogata K, Dong H, et al. Immunology of B7-H1 and its roles in human diseases. Int J Hematol 2003;78:321-8. [PubMed]
- Blank C, Gajewski TF, Mackensen A. Interaction of PD-L1 on tumor cells with PD-1 on tumor-specific T cells as a mechanism of immune evasion: implications for tumor immunotherapy. Cancer Immunol Immunother 2005;54:307-14. [PubMed]
- Blank C, Mackensen A. Contribution of the PD-L1/PD-1 pathway to T-cell exhaustion: an update on implications for chronic infections and tumor evasion. Cancer Immunol Immunother 2007;56:739-45. [PubMed]
- Blank C, Kuball J, Voelkl S, et al. Blockade of PD-L1 (B7-H1) augments human tumor-specific T cell responses in vitro. Int J Cancer 2006;119:317-27. [PubMed]
- Tsushima F, Yao S, Shin T, et al. Interaction between B7-H1 and PD-1 determines initiation and reversal of T-cell anergy. Blood 2007;110:180-5. [PubMed]
- Taube JM, Anders RA, Young GD, et al. Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med 2012;4:127ra37.