Circulating miR-499 are novel and sensitive biomarker of acute myocardial infarction
Introduction
Acute chest pain is the leading cause of hospital admission worldwide. It is critically important to accurately determine whether a patient with acute chest pain is suffering from acute myocardial infarction (AMI) without delay (1,2). Generally speaking, the diagnosis of AMI is mainly based on patient’s medical history, presenting symptoms and signs, electrocardiography (ECG) and laboratory markers. However, the patient’s medical history and sings is largely affected by the cognitive ability of the patient and the expressive ability of the clinician. For instance, some patients are unconscious when they are sent to hospitals and their family members cannot remember the exact onset time. ECG is an important basis for the diagnosis of AMI, but interpretation of the ECG is quite subjective. In addition, some AMI patients have no ECG manifestations. In contrast, laboratory markers are more favored by clinicians. Troponin (cTnI/T) and creatine kinase-MB (CK-MB) are generally accepted as the most reliable biomarkers, but neither can absolutely and accurately confirm or exclude the diagnosis of AMI (3,4). It is therefore necessary to seek more sensitive and specific novel biomarkers for the diagnosis of AMI (5).
microRNAs (miRs), non-coding single-stranded small RNA consisting of 21-23 nucleotides, are negative regulators of gene expression by binding to 3’UTR of mRNA (6,7). miRNAs used to be considered existing in cells only for some time. However, recent studies discovered that miRNAs are stably present in human serum/plasma (8,9). Most importantly, these circulating miRNAs can serve as potential biomarkers for various diseases, such as cancer (10), tissue injury (11), autoimmune diseases (12) and other common clinical diseases.
More recent studies have demonstrated that miRNAs play an important role in AMI. miR-1, miR-133a, miR-208 and microRNA-499-5p (miR-499-5p) were found to be cardio- or skeletal-muscle specific and could be used as biomarkers for the diagnosis of MI (13,14). Animal models have demonstrated that miR-499 specifically expressed in cardiomyocytes and was a potential marker of myocardial injury (15). A milder elevation with 6-fold of miRNA-499 was observed in viral myocarditis while 100-fold was observed in AMI (16), indicating that the level of plasma miR-499 was not affected by inflammation and it could reflect myocardial damage. miRNA array analysis showed that plasma miR-499 concentrations increased in AMI group while it were below the limit of detection in other patient groups (17). These clinical studies, preliminary, have indicated that miR-499 hold potential as a new cardiac biomarker.
Therefore, the present study was designed to determine the diagnostic value of circulating miR-499 in the assessment of suspected AMI patients, measure the plasma levels of cardio specific miR-499 following MI, and evaluate its usefulness as a biomarker of early cardiac cell death.
Materials and methods
Patients and subjects
A total of 227 consecutive patients with acute chest pain within 12 h admitted to departments of emergency and cardiology of Wuxi Second People’s Hospital (Wuxi, China) between October 2011 and May 2014 were recruited in the study. The inclusion criteria were based on the universal definition of MI (18). Briefly, patients with AMI were clinically diagnosed by biochemical markers (cTnI >0.1 ng/mL), acute ischemic-type chest pain, ECG change, and coronary angiography.
Patients were excluded if the chest pain caused by trauma, drugs, medical intervention, patients with terminal kidney failure who required dialysis or received intravenous thrombolytic before the initial blood samples were obtained. In addition, 100 adult healthy volunteers with normal ECG findings and no history of cardiovascular disease during the same period were enrolled as normal controls.
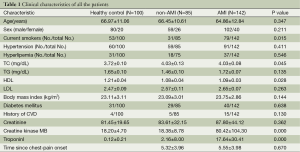
Whole blood samples (3-5 mL) were collected and extracted from citrated tubes within 2 h after hospitalization and stored at −80 °C for miRNA analysis. Finally, 0.4 mL of plasma was exactly used for RNA extraction. The protocol was approved by the local ethics committee and all study participants signed an informed consent form. Clinical characteristics are shown in Table 1.
Full table
Detection of miR-499
Total RNA was extracted from plasma samples using the mirVana PARIS kit (Ambion, Applied Biosystem, Foster City, USA) with enrichment for small RNAs. Reverse transcription of miRNA was performed using the miScript reverse transcription kit (Applied Biosystem, Inc.). MiR-499 was quantitated by TaqMan miRNA quantitative reverse transcriptase-polymerase chain reaction (qRT-PCR) assay according to the manufacturer’s instructions (Applied BioSystems, Inc.). U6RNA was used as a miRNA internal control.
Data were analyzed with automatic setting for assigning baseline. The threshold cycle (Ct) was defined as the fractional cycle number at which the fluorescence exceeds the given threshold. Ct values greater than 40 as obtained from real-time PCR were treated as 40. The plasma levels of miRNA were detected and analyzed independently by two investigators who were blinded to the clinical data of patients. Data obtained by real-time PCR were translated in log2 (relative level).
cTnI and CK-MB testing
Serum cTnI and CK-MB was assayed with BECKMAN DXI800 system and OLYMPUS AU5421 system, respectively. Both assays were performed according to the manufacturer’s instructions. The upper limit for the normal reference range was set at 0.01 ng/mL and 24 U/L, respectively.
Statistical analysis
Experimental data are expressed as mean ± standard deviation 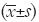 . Before analysis, all data were subjected to a normality test (Shapiro-Wilk). Kruskal-Wallis H test or one-way ANOVA was used for comparison of data between the three groups, and LSD-t test was used for inter-group comparison. Receiver operating characteristic (ROC) curves were used for evaluation of diagnostic accuracy of miR-499, CK-MB and cTnI. Spearman correlation test was used to analyze correlations between miR-499, cTnI and CK-MB. Statistical analyses were performed with the SPSS statistical package. A value P<0.05 was considered statistically significant.
. Before analysis, all data were subjected to a normality test (Shapiro-Wilk). Kruskal-Wallis H test or one-way ANOVA was used for comparison of data between the three groups, and LSD-t test was used for inter-group comparison. Receiver operating characteristic (ROC) curves were used for evaluation of diagnostic accuracy of miR-499, CK-MB and cTnI. Spearman correlation test was used to analyze correlations between miR-499, cTnI and CK-MB. Statistical analyses were performed with the SPSS statistical package. A value P<0.05 was considered statistically significant.
Results
Description and clinical features of the patients
Of the 227 patients who were initiated enrolled in this study, AMI was confirmed in 142 patients. In the non-AMI patients, stable angina was diagnosed in 13 patients, unstable angina in 18 patients, hypertensive heart disease in 18 patients, heart failure (Grade III and IV) in 10 patients, myocarditis in 4 patients, pericarditis in 2 patients, myocardial bridge in 2 patients, aortic dissection in 2 patients, pulmonary embolism in 2 patients, penetrating abdominal aortic ulcer in one patient, and ischemic cardiomyopathy in one patient. Table 1 shows clinical characteristics of all the patients.
Increased circulation concentrations of miR-499 after AMI
MiR-499 was measured in the plasma of 142 AMI patients, 85 non-AMI patients and 100 healthy controls (Figure 1A). In the healthy control, miR-499 almost had undetectable concentrations, and was at a low level in the non-MI chest pain group. Compared to the healthy control population, circulating miR-499 levels were higher in patients with AMI. Furthermore, circulating levels of miR-499 were markedly elevated in AMI patients than any subgroup of the non-AMI patients (Figure 1B).
Simultaneous miR-499 plasma levels in acute myocardial infarction patients
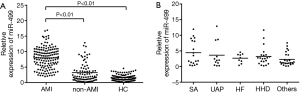
Plasma miR-499 level was measured at admission in the 142 AMI patients. Plasma miR-499 level increased gradually at different time points after the onset of chest pain. It was detectable within 1 h after the onset of chest pain and continued to increase gradually without any sign of decreasing tendency within 9 h (Figure 2). While positive CK-MB and cTnI were detected 2 and 2-4 h after chest pain respectively. Ten AMI patients were early founded by mir-499 detection who were otherwise missed by troponin and 48 by CK-MB.
Evaluation of miR-499 in plasma as new biomarkers for acute myocardial infarction
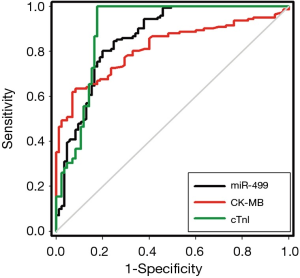
To investigate the character of miR-499 as a potential biomarker of AMI, ROC analysis was performed on data from all 227 patients with chest pain, including 142 AMI patients and 85 non-AMI patients. The ROC curves of miR-499 reflected separation between AMI and non-AMI group, with an area under curve (AUC) of 0.86 [95% confidence interval (CI), 0.81-0.91], compared with cTnI with an AUC of 0.90 (95% CI, 0.85-0.95), CK-MB with an AUC of 0.82 (95% CI, 0.76-0.87), respectively (Figure 3). These results showed that miR-499 had a diagnostic value in AMI patients, but its diagnostic accuracy was lower than that of cTnI (P<0.05).
Correlations between miR-499 with cTnI and CK-MB
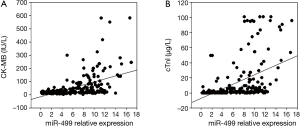
To evaluate the significance of increased levels of circulating miR-499 following myocardial infarction, we analyzed whether the levels of miR-499 correlated with clinical parameters CK-MB and cTnI. We found that the level of miR-499 correlated significantly with circulating troponinI (P=0.000, r=0.488) (Figure 4A), CK-MB (P=0.000, r=0.495) (Figure 4B).
Discussion
In the present study, our results showed that cardiac-enriched circulating miR-499 was hardly detectable in cohort of healthy controls, very close to the detection limit of the assay. However, miR-499 increased in some patients with non-AMI cardiac disease whose troponin was negative on initial presentation, including angina pectoris and myocarditis. Although the mechanisms of this phenomenon remain unclear, the packaging of miR-499 into microvesicles may be the explanation. miRNAs were considered a new type of signaling molecule, and they may have direct or indirect biological effects that can deliver multiple messages at a time or regulate numerous target genes independent of cell contact or adhesion in the same time (19,20). As a result, the release of miR-499 can be affected by any form of cellular stress including cardiac necrosis. These observations in this study suggest that miR-499 may be a new potential biomarker to distinguish angina pectoris and myocarditis from other diseases in early phases.
On the basis of previous studies, we further observed the time courses of miR-499, CK-MB and cTnI level in AMI patients with different onset time. Comparable to cTnI and CK-MB, miR-499 are present in the plasma as early as 1 h after the onset of chest pain, and continued to increase even after 9 h following the onset of chest pain, while CK-MB and cTnI were detected 2 h after chest pain, indicating that miR-499 level could be used to detect AMI earlier. This supported the evidence that miR-499 can be a very early biomarker of AMI.
Receiver operating characteristic analysis further indicated that miR-499 has considerable diagnostic efficiency. These results are partially supported by a very recent report that miR-499 level was significantly higher in plasma from AMI patients than non-AMI subjects (21).What’s more, miR-499 could early find patients missed by troponin or CK-MB. It indicates that the miR-499 profiling system may work as a potential replenishment of the currently available biomarkers for the clinical diagnosis of AMI. Based on ROC curves, we screened the cut-off values to define one that could best discriminate AMI patients from patients with non AMI chest pain, and found that miR-499 >12.93 may serve as a determinant for the diagnosis of AMI, these important cut-off values may help provide evidence for future research in this area.
Cardiac troponins are considered the gold standard of biomarkers for the diagnosis of myocardial infarction at present. MiR-499 and cTnT have a certain correlation, indicating that they can be used as markers of acute cardiomyocyte cell death. On the other hand, it suggested that the diagnostic information of miR-499 does not completely overlap with that of troponin and CK-MB, miR-499 may provide more diagnostic information than troponin. In addition, cTnT has the ability to evaluate the prognosis of AMI, so the positive correlation also indicates that it can evaluate the prognosis. A recent study elegantly demonstrated that in some patients undergoing catheterization, the concentration of several miRs (such as miR-499) in transcoronary was increased (22). So we included all patients and healthy control at the first presentation and collected blood before any treatment. We also found that plasma miR-499 could provide more diagnostic information than CK-MB and cTnI, and therefore combination of miR-499 with CK-MB and/or cTnI may improve the diagnostic accuracy.
Some problems need to be solved before miR-499 can be used as a clinical marker for AMI, including technical problems in detecting miR-499 timely. However, compared with cTnT and other conventional markers, miR-499 has unique advantages, for instance, it is not affected by renal function (21) and has high stability in vitro (14). In addition, cTnT and CK-MB are currently detected by immunological methods and susceptible to interference of some specimen factors such as giant troponin (23) and troponin antibodies (24). Thus, miR-499 is still an expected marker of myocardial injury.
In summary, this study confirms the value of circulating miR-499 as a marker for the diagnosis of AMI, devoid of the deficit of CK-MB and cTnI. It is necessary to continue with the research in this area to provide new evidence for explaining the value of miR-499 in the diagnosis and prognostic prediction of AMI.
Acknowledgements
Funding: This work was funded by Clinical technology Foundation of Jiangsu Province (BL2012042), National Natural Science Foundation of China [81301503].
Disclosure: The authors declare no conflict of interest.
References
- Kontos MC, Diercks DB, Kirk JD. Emergency department and office-based evaluation of patients with chest pain. Mayo Clin Proc 2010;85:284-99. [PubMed]
- Yeh RW, Go AS. Rethinking the epidemiology of acute myocardial infarction: challenges and opportunities. Arch Intern Med 2010;170:759-64. [PubMed]
- Chan D, Ng LL. Biomarkers in acute myocardial infarction. BMC Med 2010;8:34. [PubMed]
- Aldous SJ. Cardiac biomarkers in acute myocardial infarction. Int J Cardiol 2013;164:282-94. [PubMed]
- Mosleh W, Abdel-Qadir H, Farkouh M. Biomarkers in the emergency workup of chest pain: uses, limitations, and future. Cleve Clin J Med 2013;80:589-98. [PubMed]
- Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 2004;116:281-97. [PubMed]
- Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell 2009;136:215-33. [PubMed]
- Mitchell PS, Parkin RK, Kroh EM, et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci U S A 2008;105:10513-8. [PubMed]
- Chen X, Ba Y, Ma L, et al. Characterization of microRNAs in serum: a novel class of biomarkers for diagnosis of cancer and other diseases. Cell Res 2008;18:997-1006. [PubMed]
- Bartels CL, Tsongalis GJ. MicroRNAs: novel biomarkers for human cancer. Clin Chem 2009;55:623-31. [PubMed]
- Zhang Y, Jia Y, Zheng R, et al. Plasma microRNA-122 as a biomarker for viral-, alcohol-, and chemical-related hepatic diseases. Clin Chem 2010;56:1830-8. [PubMed]
- Carlsen AL, Schetter AJ, Nielsen CT, et al. Circulating microRNA expression profiles associated with systemic lupus erythematosus. Arthritis Rheum 2013;65:1324-34. [PubMed]
- Adachi T, Nakanishi M, Otsuka Y, et al. Plasma microRNA 499 as a biomarker of acute myocardial infarction. Clin Chem 2010;56:1183-5. [PubMed]
- Li Y, Jiang Z, Xu L, et al. Stability analysis of liver cancer-related microRNAs. Acta Biochim Biophys Sin (Shanghai) 2011;43:69-78. [PubMed]
- Wang GK, Zhu JQ, Zhang JT, et al. Circulating microRNA: a novel potential biomarker for early diagnosis of acute myocardial infarction in humans. Eur Heart J 2010;31:659-66. [PubMed]
- Corsten MF, Dennert R, Jochems S, et al. Circulating MicroRNA-208b and MicroRNA-499 reflect myocardial damage in cardiovascular disease. Circ Cardiovasc Genet 2010;3:499-506. [PubMed]
- Adachi T, Nakanishi M, Otsuka Y, et al. Plasma microRNA 499 as a biomarker of acute myocardial infarction. Clin Chem 2010;56:1183-5. [PubMed]
- Thygesen K, Alpert JS, White HD, et al. Universal definition of myocardial infarction. Eur Heart J 2007;28:2525-38. [PubMed]
- Chen X, Liang H, Zhang J, et al. Secreted microRNAs: a new form of intercellular communication. Trends Cell Biol 2012;22:125-32. [PubMed]
- Chen X, Liang H, Zhang J, et al. Horizontal transfer of microRNAs: molecular mechanisms and clinical applications. Protein Cell 2012;3:28-37. [PubMed]
- Corsten MF, Dennert R, Jochems S, et al. Circulating MicroRNA-208b and MicroRNA-499 reflect myocardial damage in cardiovascular disease. Circ Cardiovasc Genet 2010;3:499-506. [PubMed]
- De Rosa S, Fichtlscherer S, Lehmann R, et al. Transcoronary concentration gradients of circulating microRNAs. Circulation 2011;124:1936-44. [PubMed]
- Michielsen EC, Bisschops PG, Janssen MJ. False positive troponin result caused by a true macrotroponin. Clin Chem Lab Med 2011;49:923-5. [PubMed]
- Tang G, Wu Y, Zhao W, et al. Multiple immunoassay systems are negatively interfered by circulating cardiac troponin I autoantibodies. Clin Exp Med 2012;12:47-53. [PubMed]


