A meta-analysis comparing hyperfractionated vs. conventional fractionated radiotherapy in non-small cell lung cancer
Introduction
Cancer is the leading cause of death and lung cancer accounts for 1.59 million deaths worldwide (1). The estimated new cases of lung cancer in United States are 13-14% and estimated deaths are 26-28% in 2014 (mortality rate of 37.00/100, 000 in China) (1,2). Non-small cell lung cancer (NSCLC) accounts for most lung cancer and carries a 5-year survival rate of 15% (3).
The treatment of NSCLC for early stages is surgery, followed by chemotherapy with concurrent radiation for some locally advanced cancers, and palliative chemotherapy for metastatic disease (3,4). A long-term survival can be achieved with radiation therapy combined with chemotherapy in patients with locally advanced unresectable disease (3).
The overall prognosis of NSCLC is still poor. Surgery is the standard of care for patients with stage I and stage II NSCLC, but many lung cancer patients do not proceed to surgery. The reason being patients’ refusal to undergo surgery or they are judged as poor candidates to surgery, due to their age or associated medical conditions like poor cardiac or respiratory function. The surgical resection also involves a number of potential problems, including the possibility of peri-operative mortality and significant pulmonary disability (5).
An essential modality in the management of lung cancer is radiotherapy. It is used as an adjuvant treatment for stage III NSCLC to improve local control, in the postoperative setting and frequently used for the palliation of advanced and metastatic lung cancer (6). The number of fractions, the dose per fraction, the total dose and the overall duration of treatment are the main variables in a course of radiotherapy. Radiotherapists endeavour to employ a combination which will achieve the maximum tumour control with the minimum of normal tissue damage (7). During conventional radiotherapy, radiation of very large fields are used to treat the tumor with a margin and regional lymph nodes (LNs) electively (6).
In 1985, continuous hyperfractionated accelerated radiotherapy (CHART) was introduced to overcome proliferation of tumour cells during a conventional course of radiotherapy and to minimize long-term normal tissue morbility (8,9). CHART was developed by combining hyperfractionation and accelerated radiotherapy. A lower overall total dose in smaller multiple fractions per day is used in this technique. The acute tissue injury occurs only after the course is completed that can be allowed to heal and regenerate without the problem of having to complete treatment (10). CHART offers a viable alternative and reasonable treatment for those patients who are not suitable candidates for surgery (5). CHART overcomes the problem of tumour repopulation and re-oxygenation (11). The CHART weekend-less (CHARTWEL) applies the same fractionation schedule as CHART except that during the weekend no treatments are applied (12-14). Though CHART has shown a statistically significant benefit in a large multi-centre randomized controlled trial, but this technique is associated with difficulties like changing departmental working hours, and lack of financial support (10).
The data on long term use of hyperfractionated radiotherapy (HRT) (CHART or CHARTWEL) in NSCLC treatment is lacking. We performed a meta-analysis, based on published randomized trials to compare HRT vs. conventional fractionated (CF) in the treatment of NSCLC.
Materials and methods
Search strategy
A systematic search through the bibliographic databases, PubMed, Google Scholar and Cochrane Library was performed till December 2013 using the keywords (“hyperfractionated radiotherapy” or “continuous hyperfractionated accelerated radiotherapy” or “conventional radiotherapy”) and (“non-small cell lung cancer”). Reference lists of included studies and review articles were manually searched. We broadened the search range by browsing the related summary, methods and references of retrieved articles. The meta-analysis was limited to studies conducted in human.
Inclusion and exclusion criteria
The randomized trials comparing HRT or CHART with conventional radiotherapy in NSCLC were selected. The study was eligible for inclusion if (I) the study compared HRT/CHART with CF radiotherapy; (II) the subjects had inoperable NSCLC; (III) the study have clear case selection criteria; (IV) the outcome measures were overall survival (OS), local tumour control, metastasis free survival and occurrence of dysphagia after radiotherapy.
The study was excluded if (I) it was non-randomized; (II) non-comparative design; (III) compared radiotherapy with chemotherapy; (III) enrolled subjects with cancer other than NSCLC; (IV) contained previously published data.
The abstract of an article was reviewed if the title of the article and/or key words were relevant. The full text articles of all potentially relevant articles were read to consider the article for inclusion in the study. The reference lists of the included articles were cross checked to identify citations that could have been missed in the primary search steps. The articles reporting insufficient data, using non-standardized scoring systems, or lacking precise comparison methods were rejected. Two investigators reviewed the titles and abstracts, and assessed the full text to establish eligibility.
Outcomes
The primary outcome was OS at 2 and 3 years and the secondary outcomes were local tumor control, metastasis free survival and occurrence of dysphagia after radiotherapy.
OS was defined as the time from randomized to death, patients still alive were censored at the time last seen alive. Local tumour control was defined as either complete disappearance of all abnormalities in a chest radiograph/X-ray or CT or when any residual abnormality observed at 6 months remained stable for a further 6 months or more (13,15).
Dysphagia was graded as 0 (none); 1 (some discomfort on swallowing-no disturbance of diet); 2 (soft diet required); 3 (fluids only); 4 (severe difficulties even with fluids) (13).
Data extraction
The meta-analysis was reported as per the Quality of Reporting of Meta-analyses (QUOROM) statement (16). Two investigators independently assessed the quality of trials and any disagreement was resolved through discussion with the third author.
Statistical analysis
The statistical analysis was performed using software Review Manager 5.2. The output of the data is in the form of forest plot. The study heterogeneity was assessed and a P value of <0.1 was considered to be suggestive of statistical heterogeneity. A fixed effects model was used to pool data. The comparison of the effects between two groups is expressed in terms of odds ratio (OR) and its 95% confidence interval (95% CI). A fixed effect model was used because we believed that all the studies included in this analysis are functionally identical and our goal was to compute the common effect size for the identified population, and not to generalize to other populations.
Results
Trial flow
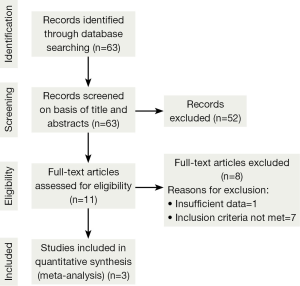
The initial search strategy retrieved a total of 63 relative studies published till December 2013. After the titles and abstracts had been reviewed, 52 papers were excluded as these did not compare HRT or CHART with conventional radiotherapy for NSCLC. The full texts of 11 articles were retrieved and read by two independent investigators. From these 11 articles identified, 8 articles were rejected because the studies did not meet inclusion criteria. One study was excluded due to insufficient data. Finally, three studies met all entry criteria and were included in the meta-analysis. The trial flow is illustrated in Figure 1.
Descriptions of studies
The characteristics of the included studies are given in Table 1. All were randomized studies and included 1,005 patients in total. The distribution of patients by sex, age, performance status, T stage, N stage, clinical stage and by histology or cytology was similar in both arms in two studies (13,15). The two studies enrolled elderly subjects with the mean age 65 (17) and 66 years (13) in both arms. One study enrolled subjects of age ranged from 31 to >71 years (15).

Full table
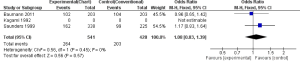
Overall survival: alive after 2 years
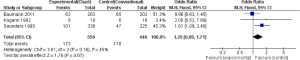
Bauman et al. reported more patients alive after 2 years (65/203) in the CF as compared to CHARTWEL (63/203), however the result was not statistical significant (0.96; 95% CI, 0.63-1.45) (13). In contrast Kagami et al. reported more patients alive after HRT (9/18) than the CF (6/18) but again the result was not statistical significant (OR, 2.00; 95% CI, 0.52-7.69) (17). However, in one study the number of subjects alive after 2 years were significantly more in CF (47/225) than the CHART (101/338) (OR, 1.61; 95% CI, 1.09-2.40) (15). When we pooled the data of these individual studies, the result significantly favored the CF over HRT (OR, 1.29; 95% CI, 0.98-1.71). This shows that HRT (CHART/CHARTWEL) did not improved OS of patients suffering from NSCLC compared with CF in 2 years (Figure 2).
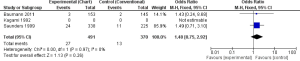
Overall survival: alive after 3 years
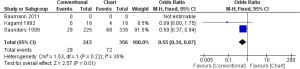
In one study (18 participants in each group), none of the patient was alive after 3 years in CF, while 4 patients were still alive in CHART/CHARTWL, though the result was not statistically significant (OR, 0.09; 95% CI, 0.00-1.75) (17). Saunders et al. found that CHART did not improve OS significantly compared to CF (OR, 0.59; 95% CI, 0.37-0.94) (18). The pooled results of the studies showed that HRT did not improve OS of patients suffering from NSLC compared with CF in 3 years, which was statistically significant (OR, 0.55; 95% CI, 0.34-0.87) (Figure 3).
Local tumour control
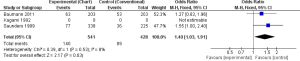
Local tumour control was more in CF than CHART/CHARTWL, however the difference in CF and CHART was statistically significant in one study (563 participants) (OR, 1.27; 95% CI, 0.83-1.96) (13) and was not statistically significant in the other study (406 participants) (OR, 1.55; 95% CI, 1.00-2.40) (17). The pooled result showed more significant tumour control in CF than CHART (OR, 1.40; 95% CI, 1.03-1.91) (Figure 4).
Metastasis free survival
Bauman et al. (14) reported high metastasis free survival in CHARTWEL (102/203) than CF (104/203), but the result was not statistically significant (OR, 0.96; 95% CI, 0.65-1.42). In contrast, Saunders et al. (15) reported high metastasis free survival in CF (99/225) than CHART (162/338), but again the result was not statistically significant (OR, 1.17; 95% CI, 0.83-1.64). However, when we pooled the result, no significant difference between the two groups was found (OR, 1.08; 95% CI, 0.83-1.39) (Figure 5).
Dysphasia
No significant difference in late dysphagia were observed in both the groups (OR, 1.48; 95% CI, 0.75-2.92) (Figure 6).
Discussion
The present meta-analysis was conducted to compare HRT (CHART/CHARTWEL) with CF for the treatment of NSCLC. Three randomized studies were identified and the data was pooled and analyzed. We compared OS at 2 and 3 years, local tumour control, and metastasis free survival and occurrence of dysphasia after radiotherapy. Overall, the results of this meta-analysis showed that HRT (CHART/CHARTWEL) was not significantly better to CF in NSCLC patients.
In our meta-analysis, one study observed no significant difference between the treatment arms for primary and secondary outcomes. OS at 2- and 3-year was not significantly different after CHARTWEL (31%, 22%) vs. CF (32%, 18%). These results indicated that CHARTWEL schedule applying 60 Gy in only 2.5 weeks did not improve OS of patients suffering from NSCLC compared to conventional radiotherapy to 66 Gy in 6.5 weeks. This is in contrast to the trial reported by Saunders et al. where OS at 2 years was 30% compared with 21% for CF. In exploratory analysis, there was no clear evidence that CHART was more or less effective in subgroups defined by sex, age, performance status, stage, treated area or histological differentiation of the squamous cell tumours. Equal number of deaths (three in each arm) that was considered to be radiation change in the lung was reported in both arms (15). Similarly in a study by Kagami et al. OS was 50% in HRT and 31.3% in CF at 2 years. They concluded that HRT can improve survival without increasing severe toxicity (17).
Bauman et al. reported that local tumour control rates and distant metastases did not differ significantly between the two arms. Acute dysphagia was more pronounced after CHARTWEL than CF (13). Similarly, Saunders et al. found similar levels of radiation morbidity in the two arms. There was no clear evidence of a difference with regard to the frequency or severity of dysphagia (18). Saunders et al. reported that HR was in favor of CF rather than CHART in the non- squamous carcinomas analysis. There was no evidence that such patients were either at a clear disadvantage or at an advantage when CHART was given. The authors suggested that further evidence to analyze the effect of CHART in non-squamous cases is required (18).
In a condition like NSCLC that has long treatment schedule, the patient satisfaction with the treatment is important. Bailey et al. conducted a study on 356 subjects (215: CHART; 141: conventional radiotherapy) to analyze the patient-completed symptom measurements in NSCLC. The results indicated relatively little overall difference between the regimens. At 1 year, no differences of >20% were seen between treatments in any symptoms. At 2 years, there was no evidence of a difference in the proportions of patients who reported “moderately” or “very much” between treatments for any symptom. The severity of sore mouth or pain on swallowing, lack of appetite, pain, and heartburn, dysphagia was more in CHART compared to CF. The largest difference was for shortness of breath, which was worse for CHART (50% vs. 27%). Significantly more patients in the CHART group reported despondent feelings (P=0.007) and constipation (P=0.041). The aim of the report was to focus on the patients’ own assessment of their symptoms. They highlighted the importance of general and psychologic symptoms, which cannot be ignored when treatment is being considered (19).
Bauman et al. observed that local tumour control was higher in CHART, but at the same time the accelerated fractionation increase acute side effects. Overall, the results of CHARTWEL do not indicate a clinically important increase of late tissue damage compared to CF. They suggested that CHARTWEL may have potential to improve outcome of sequential radio-chemotherapy. The result provides a basis for further trials on treatment intensification for locally advanced NSCLC (13).
The use of HRT and newer chemotherapy agents proposed for the NSCLC treatment are limited due to the associated severe toxicity when used in combination. King et al. demonstrated the feasibility and tolerability of high dose, accelerated hyperfractionation treatment regime in patients with NSCLC. They suggested that improvement in treatment of NSCLC may be achieved by combining chemotherapy and high-dose, accelerated, HRT, however higher rate of acute and late toxicities may be expected to be associated with such treatments (20). A study by Bonomi et al. suggested that the aggressive chemoradiation using an accelerated hyperfractionated schedule may achieve relatively good response rates to treat patients with stage III lung cancer (21). Jenkins et al. in their study found that induction chemotherapy can be safely combined with CHART and supported a continuous investigation for non-surgical alternatives in the NSCLC (22). The ultimate search for a combination of optimized radiotherapy and the most effective systemic chemotherapy in unresectable tumours provides considerable material for on-going and future clinical trials.
We comprehensively searched for randomized comparative trials from a wide range of databases in order to avoid the risk of publication bias, and used clinically relevant outcome measures. Due to a lack of relevant comparative data, the role of HRT in NSCLC is uncertain. Though many studies have reported CHART to be a better treatment option than CF for NSCLC, but the result of the current meta-analysis does not support CHART. The difference in the result may be due to small number of randomized studies included in the meta-analysis. It is not possible to draw firm conclusions as to whether HRT is better than CF. Local control is encouraging and therefore warrants further evaluation of this regimen. Evaluation of CHART/CHARTWEL in more randomized trials is urgently needed. Novel and developing radiation therapy must be incorporated as an integral part of modern cancer management. It is essential that the participation in national clinical trials is encouraged, radiotherapy techniques are optimized and combined modality approaches are fully supported (10).
Conclusions
HRT was not significantly better to conventional radiotherapy in NSCLC treatment.
Acknowledgements
Authors’ contributions: Weisan Zhang and Qian Liu conducted the statistical analysis of the data and drafted the manuscript. Xifeng Dong and Ping Lei analyzed the data with relevant medical literature and revised the manuscript. All authors read and approved the final manuscript.
Funding: This project is supported by Tianjin Health Bureu (No. 2011KZ116, No. 2010KZ106), Tianjin Natural Science Foundation (No.14JCYBJC27800, No. 10JCYBJC23700).
Disclosure: The authors declare no conflict of interest.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015;65:5-29. [PubMed]
- Zheng R, Zeng H, Zhang S, et al. Lung cancer incidence and mortality in China, 2010. Thoracic Cancer 2014;5:330-6.
- National Cancer Institute at the national Institute of Health. Non-Small Cell Lung Cancer Treatment (PDQ®). General Information About Non-Small Cell Lung Cancer (NSCLC). Available online: http://www.cancer.gov/cancertopics/pdq/treatment/non-small-cell-lung/healthprofessional, accessed on April 1, 2014.
- Molina JR, Yang P, Cassivi SD, et al. Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc 2008;83:584-94. [PubMed]
- Ghosh S, Sujendran V, Alexiou C, et al. Long term results of surgery versus continuous hyperfractionated accelerated radiotherapy (CHART) in patients aged >70 years with stage 1 non-small cell lung cancer. Eur J Cardiothorac Surg 2003;24:1002-7. [PubMed]
- Saadeddin A. Radiotherapy for NSCLC: review of conventional and new treatment techniques. J Infect Public Health 2012;5 Suppl 1:S45-9. [PubMed]
- Dische S, Saunders MI. Continuous, hyperfractionated, accelerated radiotherapy (CHART). Br J Cancer 1989;59:325-6. [PubMed]
- Dische S, Saunders MI. The rationale for continuous, hyperfractionated, accelerated radiotherapy (CHART). Int J Radiat Oncol Biol Phys 1990;19:1317-20. [PubMed]
- Saunders MI, Dische S. Radiotherapy employing three fractions in each day over a continuous period of 12 days. Br J Radiol 1986;59:523-5. [PubMed]
- Eakin RL, Saunders MI. Non-small cell lung cancer and CHART (continuous hyperfractionated accelerated radiotherapy)--where do we stand? Ulster Med J 2000;69:128-36. [PubMed]
- Sibtain A, Saunders MI, Bentzen SM, et al. Pre-treatment haemoglobin concentration in accelerated and conventional radiotherapy for non-small cell lung carcinoma. Clin Oncol (R Coll Radiol) 2004;16:58-62. [PubMed]
- Saunders MI, Rojas A, Lyn BE, et al. Experience with dose escalation using CHARTWEL (continuous hyperfractionated accelerated radiotherapy weekend less) in non-small-cell lung cancer. Br J Cancer 1998;78:1323-8. [PubMed]
- Baumann M, Herrmann T, Koch R, et al. Final results of the randomized phase III CHARTWEL-trial (ARO 97-1) comparing hyperfractionated-accelerated versus conventionally fractionated radiotherapy in non-small cell lung cancer (NSCLC). Radiother Oncol 2011;100:76-85. [PubMed]
- Baumann M, Herrmann T, Matthiessen W, et al. CHARTWEL-Bronchus (ARO 97-1): a randomized multicenter trial to compare conventional fractionated radiotherapy with CHARTWEL radiotherapy in inoperable non-small-call bronchial carcinoma. Strahlenther Onkol 1997;173:663-7. [PubMed]
- Saunders M, Dische S, Barrett A, et al. Continuous, hyperfractionated, accelerated radiotherapy (CHART) versus conventional radiotherapy in non-small cell lung cancer: mature data from the randomised multicentre trial. CHART Steering committee. Radiother Oncol 1999;52:137-48. [PubMed]
- Moher D, Cook DJ, Eastwood S, et al. Improving the quality of reports of meta-analyses of randomised controlled trials: the QUOROM statement. Quality of Reporting of Meta-analyses. Lancet 1999;354:1896-900. [PubMed]
- Kagami Y, Nishio M, Narimatsu N, et al. Prospective randomized trials comparing hyperfractionated radiotherapy with conventional radiotherapy in stage III non-small cell lung cancer. Nihon Igaku Hoshasen Gakkai Zasshi 1992;52:1452-5. [PubMed]
- Saunders MI, Dische S, Rojas A. CHART (continuous, hyperfractionated, accelerated radiotherapy): a tale of two disciplines. Br J Cancer 1999;80 Suppl 1:110-5. [PubMed]
- Bailey AJ, Parmar MK, Stephens RJ. Patient-reported short-term and long-term physical and psychologic symptoms: results of the continuous hyperfractionated accelerated [correction of acclerated] radiotherapy (CHART) randomized trial in non-small-cell lung cancer. CHART Steering Committee. J Clin Oncol 1998;16:3082-93. [PubMed]
- King SC, Acker JC, Kussin PS, et al. High-dose, hyperfractionated, accelerated radiotherapy using a concurrent boost for the treatment of nonsmall cell lung cancer: unusual toxicity and promising early results. Int J Radiat Oncol Biol Phys 1996;36:593-9. [PubMed]
- Bonomi M, Blanco-Savorio A, Cerchietti L, et al. Continuous hyperfractionated accelerated radiation therapy week-end less in combination with neoadjuvant chemotherapy for the treatment of stage III non-small-cell lung cancer. Lung Cancer 2008;60:75-82. [PubMed]
- Jenkins P, D'Amico K, Benstead K, et al. Radiation pneumonitis following treatment of non-small-cell lung cancer with continuous hyperfractionated accelerated radiotherapy (CHART). Int J Radiat Oncol Biol Phys 2003;56:360-6. [PubMed]