Pediatric heart transplantation
Introduction
Treatment of patients suffering from end-stage heart failure (HF) leaves surgeons with limited options. The failing heart may be treated for a short period of time by medications, but in the absence of other correctable diseases without either replacing the heart [heart transplantation (HTx)] or mechanical circulatory support (MCS), the destiny of the patient will take a fatal run. HTx is considered to be the “golden standard” for patients, both adults and children, suffering from end stage HF. Just three days after the world’s first human HTx, Adrian Kantrowitz performed this pioneering procedure on a 19-day-old neonate, recording the world’s second HTx. Unfortunately the infant died only hours after the operation (1). It would take another 16 years before a neonatal HTx was performed again (2). The development of cyclosporine-based immunosuppression regimens years later stimulated an increased application of HTx (3). In 2011, 565 HTx in patients below 18 years were reported to the registry of the International Society for Heart and Lung Transplantation (ISHLT); about 25% of the patients were infant recipients (4).
Special features in pediatric heart transplantation (pHTx)
Hospitalization among children suffering from HF due to congenital heart disease (CHD) is increasing (5) and pHTx may remain the only option for some of these young patients. PHTx accounts for approximately 14% of the total HTx (4). Today more than 11,000 pHTx have been reported to the registry of the ISHLT. We may examine the evolving management and outcome of this therapy. Compared to the adult population, there are some crucial differences worth mentioning. This article tries to highlight some of these differences; starting with the initial diagnosis leading to HF, donor selection, including ABO incompatible (ABOi) transplantation, waitlist management and some brief comments concerning surgical techniques.
The pediatric heart transplant recipient
Diagnoses leading to pHTx are age-specific and have also changed during the past decades. The devastating constellation of left heart anomalies summarized under the name of hypoplastic left heart syndrome (HLHS) was one of the reasons to introduce infant pHTx. In the last two decades, organ shortage especially for neonates made it clear that primary HTx was an impractical therapy for the large number of infants with HLHS (2). Attempts towards reconstructive, palliative surgery in this patient population led to staged surgery [Norwood procedure (6)] and became the primary treatment option. This explains that the percentage of recipients listed for pHTx with the diagnosis of CHD has decreased from 81% in the 1990s down to 54% in 2013 (4). Still, CHD remains the most common indication for pHTx in the infant age group (4). Out of the patients listed with CHD 60, 70% have single-ventricle physiologies. In 11 to 17 year olds, the percentage of recipients with the diagnosis of CHD decreases to 23% whereas the percentage of myopathy increases to 65% (see Table 1). The entity of cardiomyopathy (CMP) may be distributed in: 75% dilated, 12% restrictive, 8% mycoarditis and 5% hypertrophic (7). Population based studies show an incidence of CMP between 0.87 to 1.3 per 100,000 children with a median age at diagnosis of 1 year. The physicians taking care of the children with moderate to severe HF should also monitor growth of the child; besides severe HF with ventricular dysfunction, moderate HF with ventricular dysfunction with significant growth retardation should also be evaluated for pHTx. Notwithstanding the great advances made over the past several years, HTx remains a time-limited therapy. Analyses have reported an estimated half-life of 13.9 years for children after HTx (4). Especially the pediatric population is ultimately likely to be considered for re-transplantation. Nine per cent of children between 11 to 17 years are listed for re-transplantation (4). It has to be mentioned that there seems to be geographical differences as re-transplantation is most common in North America at 6% compared to only 2% in the rest of the world. Most of the re-transplantations (72%) will be made beyond three years after the primary HTx. Children with a previous transplant are likely to be sensitized, which may explain in parts that re-transplantation remains a risk factor for mortality up to 15 years post-transplant (4). Likewise, survival after re-transplantation is reduced compared with CMP but it is not significantly different from patients with CHD (4).
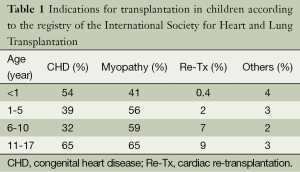
Full table
Patient selection remains a crucial factor for the outcome. Basic and specific examinations have to be conducted to exclude contraindications prior to listing (see Table 2). Neuromuscular disorders including Becker and Duchenne disease are frequently associated with the development of CMP and should be searched for. The pulmonary vascular resistance must be measured prior to listing because a fixed pulmonary hypertension (transpulmonary gradient >15 mmHg, pulmonary artery systolic pressure >55 mmHg and pulmonary vascular resistance >6 µm2) is contraindicating pHTx. Other contraindications, such as severe extra-cardiac malformations (chromosomal and genetic syndromes with poor quality of life prognosis), ongoing malignancy, have to be searched for (see Table 2). In complex congenital anatomy beside cardiac catheterization, cardiovascular imaging including computer tomography scan or magnet resonance imaging will be required. As a result of previous cardiac surgeries, pediatric heart transplant recipients are at a particular risk of sensitization affecting the postoperative immunosuppressive (IS) regime and the outcome.
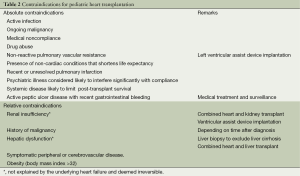
Full table
Finally, a strong reliable social support system is essential for long-term success. Family members must often restructure their daily routines to care for their children. Therefore, the child along with his/her family must undergo a complete psychosocial evaluation (8).
Awaiting pHTx
Many candidates are stable enough to be managed as outpatients. Optimal medical treatment, including diuretics, angiotensin-converting enzyme inhibitors and β-blockers, attempts to preserve end-organ function. If this fails, in-hospital treatment with intravenous inotropic support will be necessary. If despite inotropic support critical peripheral perfusion (i.e., metabolic acidosis; cardiac index <2.0 L/m2/min, mixed venous oxygen saturation <40%) with early signs of renal, hepatic or multi-organ failure occur, MCS should be considered.
The lack of suitable donor organ is displayed by the amount of patients listed for HTx in Eurotransplant (ET) countries which has doubled between 2003 and 2011, whereas the number of HTx stayed the same (9). In Switzerland the median waiting time for a donor heart has increased over the last five years from 104 to 276 days in 2013. Simultaneously there is an almost three fold increase of patients on the waitlist (Annual Swisstransplant report 2013). Especially children are at an increased risk of death on the waiting list (10). The limited numbers of available pediatric donor heart organs led to an increased mean waiting time in ET of 322 days for patients under five years of age (ET Annual report, 2012). In Switzerland the number of paediatric heart transplant candidates between 2009 and 2013 was more than four times bigger than during the 2004-2008 period (see article Weiss et al. in this issue). To prevent death while waiting, MCS support was introduced very early.
Ventricular assist devices (VADs) in children
VADs as bridge to transplantation (BTT) have become widely accepted in adult practice with excellent results (see article Pozzi et al. in this issue). There is a large variety of adult sized VADs but only a small number are available for children with a body surface area (BSA) of less than 1.2 m2 or weight less than 20 kg. Most centres have experience with extracorporeal membrane oxygenation (ECMO) as BTT; nevertheless ECMO application is limited to short-term support. Therefore VADs have also evolved over the past few years with promising outcomein the pediatric population (11-13). Crucial for successful BTT remain patient selection and timing. In patients with critical peripheral perfusion and a cardiac index <2.0 L/m2/min, despite inotropic support, MCS should be considered. There are only a few contraindications for MCS like malignant neoplastic diseases with a very limited life expectancy, advanced multi-organ failure, irreversible pulmonary failure and severe extra-cardiac malformations such as chromosomal and genetic syndromes with poor quality of life prognosis (which would also exclude them from cardiac transplantation). There is evidence that similar to adults, making decision in favor of earlier VAD implantation results in better outcome, especially in children under one year of age (14). In our own experience, MCS was initiated in 65% of recipients on the waitlist (15).
Expanding organ donation criteria
On the other side, the crucial shortage of donors has led to various approaches to improve organ availability (16) and graft utilization. To expand the donor pool, liberated organ donation criteria such as increasing age or other co-morbidities were adapted. Today, almost every fourth heart organ donor in Europe (23.2%) is 50 years of age or older (17). The proportion of adult donors allocated to pediatric recipients is 18% in the US but is as high as 43% in Europe and 48% in the rest of the world (4). Lately, the Vienna group of Eskandary et al. found no interaction between donor and recipient age negatively affecting mortality and cardiac allograft vasculopathy (CAV) (18). Others showed that older recipients, who received an older donor heart, had progressively worse survival rate (19). In the 2012 ISHLT registry report, only the donor age but not the recipient age was a risk factor for developing CAV (20). Concerning the use of donor hearts from resuscitated organ donors seems there are some hopeful publications. Using the UNOS database, Quader et al. (21) assessed the effect of donor cardiopulmonary resuscitation (CPR) on outcomes after cardiac transplant. The authors did not observe a negative effect of CPR on recipient survival at 30 days, 1 or 5 years (22). Similarly at the 2014 annual meeting of the ISHLT Khan et al. showed that donor heart organs from cardiopulmonary resuscitated donors do not decrease cardiac graft survival (abstract #97) (23).
The use of anencephalic neonates as organ donors has been discussed. Official statements from the Canadian Paediatric Society and the American Academy of Pediatrics affirmed that anencephalic infants are not appropriate organ donors. In an update of the canadian statement in 2005 it is recommended: Organ donation from anencephalic infants should not be undertaken due to the serious difficulties surrounding the establishment of brain death in these infants and the lack of evidence to date supporting successful organ transplantation. There should be no alteration or modification of standard infant brain death criteria to include infants with anencephaly (24).
An innovative strategy for the infant age group that proved to be successful was the introduction of ABOi transplantation (25).
ABO incompatible (ABOi) transplants
Infant heart transplant recipients are at a greater risk of death before transplantation than older children. The main reason is the search for an appropriately sized organ donor. This compelling motivation challenged previously unquestioned mandates such as ABO compatible transplantation. Introduction of intentional ABOi HTx was based on the immaturity of the immune system in this age group (26). Analysis has demonstrated persistent deficiency of antibodies toward the donor blood group in infants as well as absence of B-cells with specific receptors for donor blood group antigens. The stage of immunologic maturation with regard to isoagglutinin production is the main parameter to judge suitability for ABOi transplant. In the UK an ABOi transplant was performed in a 2-year-old child still without isoagglutinins (27). Preoperative anti-ABO titers of 1:4 seem to be safe. A European single center study using the Toronto protocol confirmed the good results (28). Autotransfusion devices were used perioperatively to recover autologous red cells proved to be safe to remove all circulating isohemagglutinins during the washing procedure (28). If circulating antibodies have accumulated plasma exchange has to be done directly from cardiopulmonary bypass during HTx; before opening the aortic cross clamp all circulating anti-donor antibodies must have been removed (8). Attention to ensure appropriate blood group products is essential; Plasma and platelets must be from donor blood type whereas packed erythrocyte transfusions can be of the recipient.
ABOi pHTx has led to a marked reduction in waitlist mortality in Canada and shorter time periods on the waitlist in the US (29). Recently, an international multi-center trial reported actuarial graft survival of 100% at one year, 96% at five years and 69% at 10 years post-transplant. The oldest recipient in this cohort at the time of transplant was 7.5 years (29).
Long-term outcome
Patient selection, waitlist management, quality of the organ donor heart, the surgical procedure itself and post-operative treatment will affect successful long-term outcome. Despite a higher mortality of one year, the youngest recipients experience the longest survival of all age groups with 19.7 years for infants, 16.8 years for recipients aged one to five, and 14.5 years for recipients between ages of six and 10 years (4). Causes of death depend on the time interval after HTx. In the early period, outcome is closely linked to operative mortality as well as acute graft function. Allograft rejection remains an important cause of mortality and morbidity. Medical prophylaxis and treatment (IS drugs) is necessary. In contrast to the adult population, most (71%) pediatric transplant recipients receive induction therapy; either anti-thymocyte globulin or interleukin-2 receptor antagonists. Risk analyses showed that children receiving polyclonal induction therapy have a better survival than those receiving IL2-R antagonists (4). Maintenance IS therapy comprises a three drug regime, including a calcineurin inhibitor, mycophenolate mofetil or acid and prednisolon. Most of the centers try to wean away the recipient from cortocosteroids after a certain period of time. Weaning of these patients seems to be justified by the ongoing discussion on the negative impact of IS drugs on somatic growth. So far, reports indicate sufficient growth in the children (most present with normal range height and weight scores). Still acute rejection remains a serious event, accounting for 5% of deaths even 10 years post-transplant. The worst outcome concerning rejection has recipients with treatable rejection periods within the first year after transplantation. Fortunately there is a distinct decrease of treated rejection over the last few decades (4). Recipients suffering from CHD are at a high immunological risk regarding rejection. The proportion of sensitized recipients with panel reactive antibody (PRA) is especially high (27%) in the pediatric population (4). Circulating anti-donor antibodies can result in cellular and humeral rejection; they result from previously received blood transfusions, post-gravid adolescent girls, surgical implantation of cryopreserved tissue valves, conduits, previous transplants, VADS/ECMO or even central venouse catheters. A percent of PRA greater 10% was shown to have inferior outcome compared to non-sensitized patients (8). Besides different IS regimes to treat recurrent rejection, extracorporeal photopheresis may be another promising option (30).
Chronic graft failure, known as cardiac artery vasculopathy (CAV) is one of the main risk factors for death (26.3% >10 years post-transplant). There seems to be a slower progress of CAV in infants and young children but when CAV is diagnosed, graft survival drops to about 50% after five years regardless of recipient age (4). Different risk factors for developing CAV have been reported, including donor cause of death, donor/recipient age difference, weight ratio, gender mismatch, use of no induction therapy compared to the use of induction therapy, different use of IS drugs. Treatment options are limited and heart re-transplantation is often the only option. Re-transplantation itself was found to be a risk factor for mortality in multivariate analyses (five year survival in children was 58%). Side effects of IS treatment, including chronic renal failure, increased risk of malignancy or infections determine morbidity (4). Although the greatest risk to die from infection is within the first year after pHTx infections remain a significant source of mortality and morbidity in the long-term (31). In the initial time after transplantation bacteria are the most common cause of infectious disease which changes to virus infections (i.e., CMV, EBV, Herpes simplex, Varicella zoster Parvovirus) and opportunistic infections such as Aspergillus, Pneumocvystis, Toxoplasma gondii. Contrary to older patients pediatric recipients seems to have a special high risk of lymphoma (32). While all HTx recipients are at an increased risk of malignancy post-transplant lymphoproliferative disease (PTLD) still represents the most cases in children (4). Besides age other risk factors associated with PTLD are increased frequency of rejections or use of OKT3 induction. The most important risk factor however is the Ebstein-Barr virus (EBV) serologic status. A high percentage of children are EBV negative as they do not acquire infection until adulthood; nevertheless also transplant candidates who contract EBV from the donor (even if EBV serology is positive) have an increased risk of PTLD.
Despite clinical characteristics (dialysis, ventilation, hospitalization) at the time of transplant, diagnosis and age, MCS remains a significant risk factor for mortality (4).
Operative techniques
HTx for complex CHD remains a technically challenging procedure and needs a well-trained team of surgeons, anesthesiologists, nurses, cardiologists and intensivists. Even though Ellsworth E. Wareham asserted: “Heart surgery is nothing more than a few simple steps, done well”, a transplant surgeon for pHTX needs more than just a Plan A and B and should be equipped with multiple bail out strategies. Donor heart procurement and pHTx, especially in complex CHD, need careful planning and a thorough understanding of the recipient’s anatomy. Most of the recipients have one or sometimes even numerous previous open heart surgeries. Establishing cardiopulmonary bypass in recipients with CHD should address residual defects that cannot be corrected by implantation of a normal heart (systemic and pulmonary venous anomalies, aortic arch obstruction or pulmonary branch defects), heterotaxy syndrome as well as anomalies of the systemic venous return (such as bilateral superior venae cavae) (8). Anomalous systemic venous connection has been observed in the general population about 0.1-3% and is more common with CHD (33,34).
Successful outcome will start with organ selection and organ harvesting. Selection of the donor organ has to take some aspects in considerations (8). A donor-recipient weight ratio of >2.5 and <0.5 adversely effects outcome (35). Likewise donor age might play an important role for pediatric recipients (36) whereas gender seems to have no significant impact on outcome. Finally one has to think about ischemic time; depending on the status of the recipient one has to wait for longer ischemic time versus longer waiting time including the possibility of death on the waitlist. Varying from routine donor heart harvesting, additional donor tissue additionally including the full length of the venae cavaesuperior and the innominate vein as well as the entire aortic transverse arch and the branch pulmonary arteries should be explanted.
“Univentricular hearts” and “Failing Fontan”
Reconstructive surgery, known as Norwood stage 1 to 3 or Fontan procedure, is a successful palliation in patients with single-ventricle physiology. Nowadays, HTx is reserved for those few newborns who do not seem suitable for the ultimate Fontan physiology (2). In neonates with HLHS the aortic arch is too small for aortic cannulation for cardiopulmonary bypass. In these cases the main pulmonary artery and the ductus can be used. Another option may be to suture a graft to the innominate artery. If HTx is done after the bidirectional cavopulmonary connection (Norwood stage II) has failed due to pulmonary resistance and the Fontan completion (Norwood stage III) is not possible, a takedown of the Glenn ansatomosis and cavo-caval transplantation is required.
After the Fontan completion late hemodynamic complications like HF, cyanosis and protein-losing enteropathy occure patients should be evaluated for HTx. Often more than one factor may led to transplantation i.e., significant cyanosis limiting exercise capacity and failing Fontan physiology (37). Besides marginal liver function, coagulopathy, several previouse heart operations the distorted anatomy may be a great challenge for the surgical team. The post-transplant survival in this patient population seems to be good but there are some high-risk factors like younger patients age who had not had the Fontan operation or who were <6 months from their Fontan procedure and ventilator dependent recipients (38).
Describing the multitude of different techniques to address cardiac transplantation in complex CHD would go beyond the scope of this article. Besides all the adversities, “the real trump card of surgery is the fact that the native organ is being removed” (8).
Congenital heart disease (CHD) in adulthood
The population of adult CHD patients is growing over the last several years due to improved survival through childhood (17). It is estimated that 10-20% of patients suffering from complex CHD will require HTx at some time of her/his life. They are at a higher risk of mortality due to myocardial dysfunction and HF (39). In an analysis of the UNOS database, Gelow et al. showed that CHD patients are less likely to receive an allograft while listed, are less likely to receive VAD therapy and more likely to die on the waitlist (40). In contrast, favorable long-term survival after HTx has been published for this patient population (41-43). These patients pose new challenges to transplant therapy.
Conclusions
pHTx has evolved over the years to a well-established, live saving procedure with excellent long-term outcome. In the next years, adults with CHD, new IS regiments and avoidance of long-term morbidity after pHTx will be just some of the issues to focus on.
Acknowledgements
We would like to acknowledge all colleagues involved in the field of pediatric heart transplantation of the pediatric heart centre Zurich.
Disclosure: The authors declare no conflict of interest.
References
- Kantrowitz A, Haller JD, Joos H, et al. Transplantation of the heart in an infant and an adult. Am J Cardiol 1968;22:782-90. [PubMed]
- Bailey LL. Origins of neonatal heart transplantation: an historical perspective. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu 2011;14:98-100. [PubMed]
- Starnes VA, Stinson EB, Oyer PE, et al. Cardiac transplantation in children and adolescents. Circulation 1987;76:V43-7. [PubMed]
- Dipchand AI, Kirk R, Edwards LB, et al. The Registry of the International Society for Heart and Lung Transplantation: Sixteenth Official Pediatric Heart Transplantation Report--2013; focus theme: age. J Heart Lung Transplant 2013;32:979-88. [PubMed]
- Adachi I, Fraser CD Jr. Mechanical circulatory support for infants and small children. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu 2011;14:38-44. [PubMed]
- Norwood WI, Lang P, Hansen DD. Physiologic repair of aortic atresia-hypoplastic left heart syndrome. N Engl J Med 1983;308:23-6. [PubMed]
- Canter CE, Shaddy RE, Bernstein D, et al. Indications for heart transplantation in pediatric heart disease: a scientific statement from the American Heart Association Council on Cardiovascular Disease in the Young; the Councils on Clinical Cardiology, Cardiovascular Nursing, and Cardiovascular Surgery and Anesthesia; and the Quality of Care and Outcomes Research Interdisciplinary Working Group. Circulation 2007;115:658-76. [PubMed]
- Canter CE, Kirklin JK. eds. ISHLT Monograph Series Volume 2: Pediatric Heart Transplantation, 1st Edition. Philadelphia: Elsevier, 2007.
- Wilhelm MJ, Ruschitzka F, Falk V. Destination therapy--time for a paradigm change in heart failure therapy. Swiss Med Wkly 2013;143:w13729. [PubMed]
- Almond CS, Thiagarajan RR, Piercey GE, et al. Waiting list mortality among children listed for heart transplantation in the United States. Circulation 2009;119:717-27. [PubMed]
- Fraser CD Jr, Jaquiss RD, Rosenthal DN, et al. Prospective trial of a pediatric ventricular assist device. N Engl J Med 2012;367:532-41. [PubMed]
- Morales DL, Almond CS, Jaquiss RD, et al. Bridging children of all sizes to cardiac transplantation: the initial multicenter North American experience with the Berlin Heart EXCOR ventricular assist device. J Heart Lung Transplant 2011;30:1-8. [PubMed]
- Schweiger M, Dave H, Lemme F, et al. Paediatric ventricular assist devices: current achievements. Swiss Med Wkly 2013;143:w13804. [PubMed]
- Potapov EV, Stiller B, Hetzer R. Ventricular assist devices in children: current achievements and future perspectives. Pediatr Transplant 2007;11:241-55. [PubMed]
- Freya S, Prêtre R, Stiasnya B, et al. Morbidity and mortality in children and adolescents listed for heart transplantation. Cardiovasc Med 2011;14:176-81.
- Schweiger M, Wasler A, Prenner G, et al. Improving the rate of organ donation. Transplant Proc 2004;36:2543-5. [PubMed]
- Stehlik J, Edwards LB, Kucheryavaya AY, et al. The Registry of the International Society for Heart and Lung Transplantation: Twenty-eighth Adult Heart Transplant Report--2011. J Heart Lung Transplant 2011;30:1078-94. [PubMed]
- Eskandary FA, Kohl M, Dunkler D, et al. Lack of donor and recipient age interaction in cardiac transplantation. J Heart Lung Transplant 2014;33:629-35. [PubMed]
- Lund LH, Edwards LB, Kucheryavaya AY, et al. The Registry of the International Society for Heart and Lung Transplantation: Thirtieth Official Adult Heart Transplant Report--2013; focus theme: age. J Heart Lung Transplant 2013;32:951-64. [PubMed]
- Stehlik J, Edwards LB, Kucheryavaya AY, et al. The Registry of the International Society for Heart and Lung Transplantation: 29th official adult heart transplant report--2012. J Heart Lung Transplant 2012;31:1052-64. [PubMed]
- Quader MA, Wolfe LG, Kasirajan V. Heart transplantation outcomes from cardiac arrest-resuscitated donors. J Heart Lung Transplant 2013;32:1090-5. [PubMed]
- DePasquale EC, Schweiger M, Ross HJ. A contemporary review of adult heart transplantation: 2012 to 2013. J Heart Lung Transplant 2014;33:775-84. [PubMed]
- Schweiger M, Huebler M. ISHLT 2014: thoracic transplant and ventricular assist devices in children—highlights of interest. Transl Pediatr 2014;3:E1-2.
- Use of anencephalic newborns as organ donors. Paediatr Child Health 2005;10:335-7. [PubMed]
- West LJ, Pollock-Barziv SM, Dipchand AI, et al. ABO-incompatible heart transplantation in infants. N Engl J Med 2001;344:793-800. [PubMed]
- West LJ. B-cell tolerance following ABO-incompatible infant heart transplantation. Transplantation 2006;81:301-7. [PubMed]
- Rao JN, Hasan A, Hamilton JR, et al. Abo-incompatible heart transplantation in infants: the Freeman Hospital experience. Transplantation 2004;77:1389-94. [PubMed]
- Daebritz SH, Schmoeckel M, Mair H, et al. Blood type incompatible cardiac transplantation in young infants. Eur J Cardiothorac Surg 2007;31:339-43; discussion 343. [PubMed]
- Urschel S, Larsen IM, Kirk R, et al. ABO-incompatible heart transplantation in early childhood: an international multicenter study of clinical experiences and limits. J Heart Lung Transplant 2013;32:285-92. [PubMed]
- Carlo WF, Pearce FB, George JF, et al. Single-center experience with extracorporeal photopheresis in pediatric heart transplantation. J Heart Lung Transplant 2014;33:624-8. [PubMed]
- Canter C, Naftel D, Caldwell R, et al. Survival and risk factors for death after cardiac transplantation in infants. A multi-institutional study. The Pediatric Heart Transplant Study. Circulation 1997;96:227-31. [PubMed]
- Opelz G, Döhler B. Lymphomas after solid organ transplantation: a collaborative transplant study report. Am J Transplant 2004;4:222-30. [PubMed]
- De Leval MR, Ritter DG, McGoon DC, et al. Anomalous systemic venous connection. Surgical considerations. Mayo Clin Proc 1975;50:599-610. [PubMed]
- Nsah EN, Moore GW, Hutchins GM. Pathogenesis of persistent left superior vena cava with a coronary sinus connection. Pediatr Pathol 1991;11:261-9. [PubMed]
- Boucek MM, Waltz DA, Edwards LB, et al. Registry of the International Society for Heart and Lung Transplantation: ninth official pediatric heart transplantation report--2006. J Heart Lung Transplant 2006;25:893-903. [PubMed]
- Chin C, Miller J, Robbins R, et al. The use of advanced-age donor hearts adversely affects survival in pediatric heart transplantation. Pediatr Transplant 1999;3:309-14. [PubMed]
- Jayakumar KA, Addonizio LJ, Kichuk-Chrisant MR, et al. Cardiac transplantation after the Fontan or Glenn procedure. J Am Coll Cardiol 2004;44:2065-72. [PubMed]
- Bernstein D, Naftel D, Chin C, et al. Outcome of listing for cardiac transplantation for failed Fontan: a multi-institutional study. Circulation 2006;114:273-80. [PubMed]
- Nieminen HP, Jokinen EV, Sairanen HI. Causes of late deaths after pediatric cardiac surgery: a population-based study. J Am Coll Cardiol 2007;50:1263-71. [PubMed]
- Gelow JM, Song HK, Weiss JB, et al. Organ allocation in adults with congenital heart disease listed for heart transplant: impact of ventricular assist devices. J Heart Lung Transplant 2013;32:1059-64. [PubMed]
- Bhama JK, Shulman J, Bermudez CA, et al. Heart transplantation for adults with congenital heart disease: results in the modern era. J Heart Lung Transplant 2013;32:499-504. [PubMed]
- Karamlou T, Hirsch J, Welke K, et al. A United Network for Organ Sharing analysis of heart transplantation in adults with congenital heart disease: outcomes and factors associated with mortality and retransplantation. J Thorac Cardiovasc Surg 2010;140:161-8. [PubMed]
- Patel ND, Weiss ES, Allen JG, et al. Heart transplantation for adults with congenital heart disease: analysis of the United network for organ sharing database. Ann Thorac Surg 2009;88:814-21; discussion 821-2. [PubMed]


