Implementation of a pulmonary thromboembolism prophylaxis program in Chinese lung surgery patients: compliance and effectiveness
Introduction
Postoperative pulmonary embolism (PE) can be a devastating postoperative complication and a leading cause of mortality after thoracic surgery (1-3). Although the American College of Chest Physicians (ACCP) has published guidelines for prevention of venous thromboembolism (VTE) in non-orthopedic surgical patients in 2012 (4-6), the VTE prophylaxis was not fully implemented all over the world, at least in China (7). The ACCP guidelines include mechanical prophylaxis for low-risk or very low-risk patients, chemoprophylaxis or mechanical prevention for middle-risk patients and chemoprophylaxis combined with mechanical prevention for high-risk patients (6). The assessment of such risk often involves the Caprini VTE risk assessment model (RAM), championed by Joseph Caprini (8-10) and others (11,12). A program of early postoperative mobilization and prophylaxis based on an adjusted Caprini RAM has been applied in thoracic patients at Boston Medical Center (BMC) and found to significantly reduce the likelihood of VTE complication after surgery (13).
However, more than half (53.91%) of surgeons in China do not follow VTE prophylaxis guidelines after lung cancer resection, and continue to make decisions on the method and duration of VTE prophylaxis depending on their own clinical experience only (14). On the other hand, it is not known whether following guidelines designed for Western populations is necessarily beneficial in Chinese populations. It is possible that Chinese patients may require different VTE prophylaxis practices. PE actually is an uncommon event in the Asian population judging from the Japanese report (15). In this report, death from PE was observed in only two patients among 30,597 (0.007%) lung cancer cases undergoing lobectomy in Japan. In our own hospital, we have observed a lower overall PE incidence in patients after lung cancer resection compared to Western reports (4,16) and hence a lower dose of thrombolytic treatment has traditionally been used for massive or sub-massive PE compared to Western practices (17-20). Therefore, we hypothesized that preventative measures for postoperative PE specific to Chinese patients may be needed which are distinct from Western guidelines. In this study, we developed a simple prophylaxis program against pulmonary thromboembolism after lung surgery using the Caprini RAM for risk stratification. Our objective was to investigate if such a program may contribute to lowering the risk of PE, and whether it could be implemented with good compliance in a Chinese hospital. We present the following article in accordance with the STROBE guideline checklist (available at http://dx.doi.org/10.21037/jtd-20-690).
Methods
Patients
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). All consecutive adult patients who received lung resection surgery for lung cancer or inflammatory lung disease by the Department of Thoracic Surgery of Shanghai Pulmonary Hospital were eligible for this study. Informed consent was obtained at the time of admission. The cohort enrollment began on 8 August 2017 and ended at 12 September 2017. Inclusion criteria included: adult patients able to give informed consent; major lung resection performed via open or minimally invasive surgical approaches; and surgery with curative intent for lung cancer or inflammatory lung diseases. Exclusion criteria included: history of inferior vena cava filter placement; history of anti-coagulation treatment; presence of deep venous thrombosis (DVT) found during the preoperative check-up; and those patients lost to follow-up. Routine lower limb Doppler ultrasonography was performed in all patients before surgery, and those diagnosed with DVT were referred for further treatments before surgery.
The Caprini RAM
All patients were scored using a Caprini RAM (10-13,21). The variables included age, body mass index (BMI), surgery, malignancy, previous medical history, existing symptoms, and so on. We omitted consideration of the followings because of technical limitations and their rarity in Chinese patients: Prothrombin 20210A; factor V Leiden; lupus anticoagulant; and anticardiolipin antibodies. The Caprini score of each patient was assessed and recorded by two attending ICU doctors at 8 a.m. of the first postoperative day (POD1). Then the patients were classified into low (0–4) or high (≥5) PE risk level based on the Caprini score.
Postoperative thromboprophylaxis protocol
Our prophylaxis program for PE was initiated on 8 August 2017. Before that time, thromboprophylaxis measures—namely intra-operative management and pre-operative low-molecular weight heparin (LMWH)—were only used sporadically according to each surgeon’s own preferences. With implementation of our program, all postoperative patients were admitted to the thoracic postoperative intensive care unit (ICU) immediately after surgery and were observed there for at least 24 hours. Our prophylaxis program included two elements: (I) early ambulation defined as mobilizing out of bed started no later than 24 hours after surgery; (II) early chemoprophylaxis with LMWH injection subcutaneously once daily, started no later than 24 hours after surgery. Early ambulation alone was used for patients at low risk (Caprini 0–4), early chemoprophylaxis plus early ambulation was for patients at high risk (Caprini ≥5). Patients who for whatever reason only received LMWH after discharge from ICU back to the general ward (i.e., after 24 hours from surgery) were defined as having received late chemoprophylaxis. Use of the program in any patient is postponed or cancelled if any of the following occurred: total chest tube drainage of more than 500 mL within 24 hours after surgery; major bleeding encountered during operation; surgeon in charge identified any clinical contraindication (such as development of a thoracic hematoma). All chemoprophylaxis treatment was used only during hospitalization, and no outpatient PE prophylaxis was prescribed after discharge.
Data collection and patient follow-up
Data for all patients were prospectively collected after surgery. In addition, patients were followed up twice at 30 and 60 days after surgery by phone call. At any time during in-hospital stay, PE-associated symptoms or clinical signs were identified, including chest pain, shortness of breath, hemoptysis, cyanosis, decrease of SpO2 (oxyhemoglobin saturation by pulse oximeter) or unexplained blood pressure decrease. If these were identified, the patient would receive computed tomographic pulmonary angiography (CTPA). Then if PE was diagnosed, the patient would receive anti-coagulation or thrombolytic treatment according to hospital guidelines. During the follow up, the patients were requested if they had some problems of chest pain, shortness of breath and if they had visited local doctors routinely and what about the routine test results. Our nurses requested the patients over phone and recorded the answers in the formal questionnaire.
Compliance analysis
To assess risk factors for non-compliance with the prophylaxis program, we used multivariable logistic regression with age, sex, surgical procedures, pathology, postoperative chest drainages included. Then we performed propensity-score matching with the identified variables to pair on a 1:1 bases patients in the high-risk group in whom the prophylaxis was used according to the program (compliance) with those in whom the program was not followed (non-compliance).
Statistical analysis
We used SPSS software (IBM Corp. Released 2017. IBM SPSS Statistics for Windows, Version 25.0. Armonk, NY: IBM Corp.) to perform statistical analysis. Results were presented as means ± standard deviation (SD). The independent-samples t-test was used for continuous variables. The Chi-square test and the Fisher’s exact test was used for categorical variables. Spearman correlation and multivariable logistic regression was performed for prediction analyses and to explain the relationship between variables. A P value <0.05 was considered statistically significant.
Results
Patients
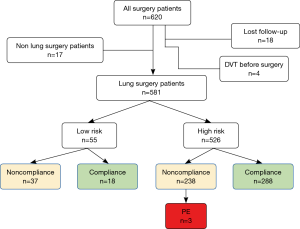
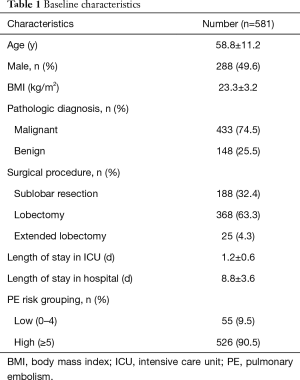
In total, 620 postoperative patients were identified as potentially eligible for study from August 8 to September 12 of 2017 (Figure 1). Exclusions included: 17 patients who received non-lung surgery; 18 patients lost during follow-up, and 4 patients found to have DVT before surgery. Finally 581 patients were enrolled. As shown in Table 1, sub-lobar resection (n=188), lobectomy (n=368) and extended lobectomy (n=25) were performed for lung cancer or inflammatory lung diseases in 433 (74.5%) and 148 (25.5%) cases respectively. Of 581 lung surgery patients, 55 cases were stratified as low PE risk level, 526 cases were high PE risk level.
Full table
At follow-up up to 60 days after surgery, three patients (0.52%) were found to have developed PE. All three patients developed PE during their postoperative hospitalization. These three PE cases all belonged to high risk group. In all three patients, there was non-compliance with the prophylaxis program: none was administered with early chemoprophylaxis, and two of them performed early ambulation. As for treatment, one patient (Caprini 6) received low-dose thrombolytic treatment, but died. The other two patients (Caprini 9 and 10, respectively) were alive, and received anti-coagulation treatment with LMWH twice a day. No major bleeding occurred with the use of chemoprophylaxis in all patients in this study.
Compliance versus non-compliance
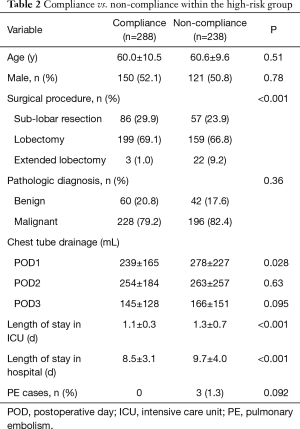
The rate of compliance with the program was 52.7% (306/581) in the entire cohort. Among 55 patients in the low risk group, only 18 (32.7%) had full compliance with our protocol, but PE did not occur in this group. Amongst patients in the high-risk group, 288 (54.8%) had full compliance with the program, but 238 (45.2%) did not. As shown in Table 2, the rate of PE was 0% in the full compliance subgroup, compared to 1.3% in the non-compliance subgroup (P=0.092). Within the high-risk group, the occurrence of PE was related to non-compliance (r=0.08, P=0.056).
Full table
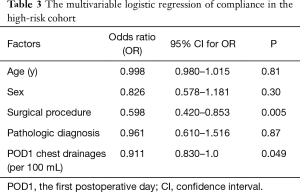
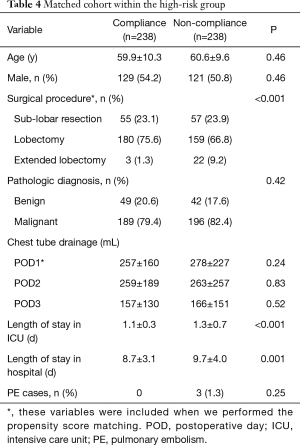
Patients in the non-compliance subgroup of high-risk patients had: higher frequency of extended lobectomy (9.2% vs. 1.0%, P<0.001); higher volume of chest drainage on POD1 (mean of 278 vs. 239 mL, P=0.028); longer duration of ICU stay (mean of 1.3 vs. 1.1 days, P<0.001); and longer overall hospital stay (mean of 9.7 vs. 8.5 days, P<0.001) (Table 2). There were no differences in the age, sex and pathologic diagnosis distribution between the compliance and non-compliance subgroups. Multivariable logistic regression for the high-risk group showed that the main factors influencing compliance with the program were: surgical procedure and POD1 chest drainage (Table 3).
Full table
Next, we would like to balance these two factors within high risk group. We performed propensity score matching with surgical procedure and chest drainage included and paired 238 patients in the compliance subgroup with 238 in the non-compliance subgroup. The standardized mean difference before matching was 0.025, and after matching it was 0.013. After matching, we found that non-compliance was linked to: surgical procedure performed; length of stay in ICU; and length of in-patient hospital stay (Table 4).
Full table
Discussion
It has been more than 5 years since the ACCP published the prevention guidelines for VTE in non-orthopedic surgical patients (6). However, in China, similar guidelines for PE in thoracic surgical patients have not emerged (14). In this study, we adopted the Caprini RAM for stratifying PE risk in Chinese lung surgery patients, and hence to allocate them to receive a new, simple-to-follow PE prophylaxis program. We found an overall rate of PE of 0.52% in our series. However, the rate of compliance with using the program was poor at only 52.7% overall. In comparison, a study from the US reported a compliance rate of 96% and the PE incidence was 2.3% (13).
The low rate of PE amongst Chinese patients, also Japanese patients, after major surgery has been observed and speculated upon before (15-17). Our study appears to corroborate this. But it is still possible that our study may have underestimated the rate of actual PE as investigations were only done in patients developing symptoms or signs suggestive of PE. Also, we only considered PE in this study, although postoperative VTEs may include DVT without embolism to the chest. It has indicated that routine lower limb Doppler ultrasonography before patients were discharged might provide a different picture of VTE incidence in thoracic surgery patients (22).
The low rate of compliance is more striking. However, it is difficult to say exactly what the reason for such low compliance may be. The probable major reason in this study was the extent of the surgical procedures which was consistent with other studies (23). We note that the volume of POD1 chest drainage tended to be higher in the non-compliance subgroup than in the compliance subgroup. It is possible that surgeons in our hospital may approach postoperative drainage volumes with greater conservatism in patients after major lung surgery, and may be more reluctant to use LMWH in patients with relatively higher drainage. It is noted that non-compliance was also correlated with a longer ICU stay and a longer overall hospital stay. It is difficult to be certain in an observational study such as this whether the poor compliance was the cause or the result of any events that led to longer stays. It is entirely possible that multiple factors—including surgical training and culture—may also play a role. While in another study also from US which is quite similar with ours, the compliance rate was 60.5% and only 1 case occurred PE among 522 patients (23). They draw a conclusion that their prophylaxis program was safe and feasible. Although we had a lower compliance rate, PE incidence correlated with non-compliance in a very close to significance P value, we can also deem that our prophylaxis program was effective.
The importance of compliance, however, seems to be reaffirmed by this study. In Chinese patients with high risk for PE after lung surgery, non-compliance with the prophylaxis is now confirmed to be linked to higher rate of postoperative PE, even though the overall rate is lower than in the West. This further strengthens the argument that VTE prophylaxis needs to be implemented in Chinese lung surgery patients, and better effort should be made to ensure good compliance.
A non-random design of this study was the main limitation. Ideally, the effect of the prophylaxis program should be assessed by randomizing patients to receive or not receive it. In this study, the separation of patients into groups who did or did not receive prophylaxis was done by considering whether or not the program was fully followed (compliance versus non-compliance). Although this still achieved the result that there were two groups of fairly evenly matched patients who did or did not receive the program, the compliance/non-compliance dichotomization in our study potentially introduces confounding variables that may have biased who received the program fully and who did not. We performed propensity-score matching to try to minimize this concern, but we acknowledge that even this is not a substitute for a future randomized study.
In summary, our study demonstrated that implementing a PE prophylaxis program for lung surgery patients in China contributed to lowering the risk of PE but may be hindered by a low rate of compliance. Nonetheless, failure of compliance in patients with high risk for PE after lung surgery may be linked to worse outcomes, and hence there is a real need to develop and enforce such a program.
Acknowledgments
We would like to give thanks to all the nursing staff for their hard work during the program implementation.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/jtd-20-690
Data Sharing Statement: Available at http://dx.doi.org/10.21037/jtd-20-690
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd-20-690). ADLS serves as an unpaid editorial board member of Journal of Thoracic Disease from Jul 2018 to Jun 2020. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of Shanghai Pulmonary Hospital. The Institutional Review Board approved submission and publication of this work and informed patient consent was obtained. The date of the approval was May 15 of 2017, the ID number was K17-123.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Dentali F, Malato A, Ageno W, et al. Incidence of venous thromboembolism in patients undergoing thoracotomy for lung cancer. J Thorac Cardiovasc Surg 2008;135:705-6. [Crossref] [PubMed]
- Bagaria V, Modi N, Panghate A, et al. Incidence and risk factors for development of venous thromboembolism in Indian patients undergoing major orthopaedic surgery: results of a prospective study. Postgrad Med J 2006;82:136-9. [Crossref] [PubMed]
- Rotman JA, Plodkowski AJ, Hayes SA, et al. Postoperative complications after thoracic surgery for lung cancer. Clin Imaging 2015;39:735-49. [Crossref] [PubMed]
- Gomez-Hernandez MT, Rodriguez-Perez M, Novoa-Valentin N, et al. Prevalence of venous thromboembolism in elective thoracic surgery. Arch Bronconeumol 2013;49:297-302. [Crossref] [PubMed]
- Reinke CE, Karakousis GC, Hadler RA, et al. Incidence of venous thromboembolism in patients undergoing surgical treatment for malignancy by type of neoplasm: An analysis of ACS-NSQIP data from 2005 to 2010. Surgery 2012;152:186-92. [Crossref] [PubMed]
- Gould MK, Garcia DA, Wren SM, et al. Prevention of VTE in nonorthopedic surgical patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012;141:e227S-e277S.
- Cui S, Li H, Tian B, et al. Risk Factors Associated with Venous Thromboembolism after Lung Cancer Surgery: A Single-center Study. Zhongguo Fei Ai Za Zhi 2018;21:753-60. [PubMed]
- Caprini JA. Thrombosis risk assessment as a guide to quality patient care. Dis Mon 2005;51:70-8. [Crossref] [PubMed]
- Bahl V, Hu HM, Henke PK, et al. A validation study of a retrospective venous thromboembolism risk scoring method. Ann Surg 2010;251:344-50. [Crossref] [PubMed]
- Caprini JA. Risk assessment as a guide to thrombosis prophylaxis. Curr Opin Pulm Med 2010;16:448-52. [Crossref] [PubMed]
- Cassidy MR, Rosenkranz P, McAneny D. Reducing postoperative venous thromboembolism complications with a standardized risk-stratified prophylaxis protocol and mobilization program. J Am Coll Surg 2014;218:1095-104. [Crossref] [PubMed]
- Hachey KJ, Hewes PD, Porter LP, et al. Caprini venous thromboembolism risk assessment permits selection for postdischarge prophylactic anticoagulation in patients with resectable lung cancer. J Thorac Cardiovasc Surg 2016;151:37-44.e1. [Crossref] [PubMed]
- Hachey KJ, Sterbling H, Choi DS, et al. Prevention of Postoperative Venous Thromboembolism in Thoracic Surgical Patients: Implementation and Evaluation of a Caprini Risk Assessment Protocol. J Am Coll Surg 2016;222:1019-27. [Crossref] [PubMed]
- Song CF, Li H, Tian B, et al. Survey of current status of prevention of venous thromboembolism after thoracic surgery in China. Zhonghua Wai Ke Za Zhi 2017;55:661-6. [PubMed]
- Committee for Scientific Affairs, The Japanese Association for Thoracic Surgery, Shimizu H, et al. Thoracic and cardiovascular surgery in Japan in 2016: Annual report by The Japanese Association for Thoracic Surgery. Gen Thorac Cardiovasc Surg 2019;67:377-411. [Crossref] [PubMed]
- Shen L, Li Y, Hernandez-Arenas LA, et al. Successful treatment of a pulmonary embolism with low dose of tissue plasminogen activator after thoracic surgery. Am J Emerg Med 2016;34:2259.e5-e6. [Crossref] [PubMed]
- Wang C, Zhai Z, Yang Y, et al. Efficacy and safety of low dose recombinant tissue-type plasminogen activator for the treatment of acute pulmonary thromboembolism: a randomized, multicenter, controlled trial. Chest 2010;137:254-62. [Crossref] [PubMed]
- Barrett NA, Byrne A, Delaney A, et al. Management of massive pulmonary embolism: a retrospective single-centre cohort study. Crit Care Resusc 2010;12:242-7. [PubMed]
- Torbicki A, Perrier A, Konstantinides S, et al. Guidelines on the diagnosis and management of acute pulmonary embolism: the Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology (ESC). Eur Heart J 2008;29:2276-315. [Crossref] [PubMed]
- Uresandi F, Monreal M, Garcia-Bragado F, et al. National Consensus on the Diagnosis, Risk Stratification and Treatment of Patients with Pulmonary Embolism. Spanish Society of Pneumology and Thoracic Surgery (SEPAR). Society Espanola Internal Medicine (SEMI). Spanish Society of Thrombosis and Haemostasis (SETH). Spanish Society of Cardiology (ESC). Spanish Society of Medicine Accident and Emergency (SEMES). Spanish Society of Angiology and Surgery Vascular (SEACV). Arch Bronconeumol 2013;49:534-47. [PubMed]
- Sterbling HM, Rosen AK, Hachey KJ, et al. Caprini Risk Model Decreases Venous Thromboembolism Rates in Thoracic Surgery Cancer Patients. Ann Thorac Surg 2018;105:879-85. [Crossref] [PubMed]
- Tian B, Li H, Cui S, et al. A novel risk assessment model for venous thromboembolism after major thoracic surgery: a Chinese single-center study. J Thorac Dis 2019;11:1903-10. [Crossref] [PubMed]
- Laws A, Anderson K, Hu J, et al. Implementation of a Venous Thromboembolism Prophylaxis Protocol Using the Caprini Risk Assessment Model in Patients Undergoing Mastectomy. Ann Surg Oncol 2018;25:3548-55. [Crossref] [PubMed]