Intrathoracic desmoid tumor arising at a distance from thoracotomy sites after thoracoscopic segmentectomy: report of a case
Introduction
Desmoid tumors are fibroblastic proliferations originating from deep soft tissue and are relatively rare neoplasms. These tumors seldom metastasize to distant areas, but they are characterized by invasive growth and may require wide resection (1). Desmoid tumors appear in various generations and can develop after injury or surgery. It has recently been reported that an intrathoracic desmoid tumor can arise after thoracotomy or even after thoracoscopic surgery (2,3). Herein, we report a case of an intrathoracic desmoid tumor in a 68-year-old woman who underwent video-assisted thoracoscopic right basal segmentectomy for lung cancer 1 year earlier. The tumor developed away from the thoracoscopic wound. The etiology is discussed.
Case report
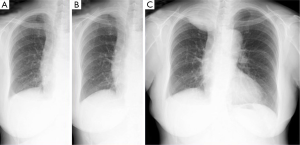
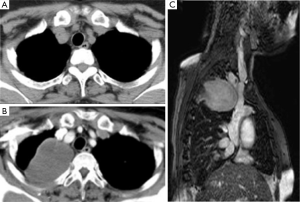
A 68-year-old woman underwent right basal segmentectomy and lymphadenectomy for lung adenocarcinoma by means of video assisted thoracoscopic surgery. The pathologic stage was T1aN0M0 stage IA as categorized using the UICC 7th classification. The 7 cm long utility window was made at the fifth intercostal space and two ports were made at the seventh intercostal space. Double chest drainage tubes with a size of 24 Fr were inserted into the apical and supra-phrenic spaces. Both drainage tubes were left in place until postoperative day 4. The patient was uneventfully discharged on postoperative day 8. A chest X-ray taken at 9 months after surgery showed a right apical mass and the tumor had rapidly enlarged on the chest X-ray taken at 1 year after surgery (Figure 1). There was no antecedent history of trauma or other chest surgery. The patient complained of dull pain in the right shoulder with an increase in the size of the tumor. A computed tomography scan demonstrated new development of a soft tissue tumor in the right apex of the lung after the initial surgery (Figure 2A,B). Magnetic resonance imaging revealed a heterogeneous tumor of medium intensity relative to the muscle on T1-weighted images and invasion to the intercostal muscles. T2-weighted images exhibited high intensity (Figure 2C). Positron emission tomography with 18F-fluorodeoxyglucose (FDG) showed FDG accumulation in the tumor. The maximum standardized uptake value was 4.05. Exploratory thoracoscopy for definite diagnosis suggested the tumor was not lung cancer recurrence, but a demarcated soft tissue tumor arising from the chest wall. A right postero-lateral thoracotomy for complete resection was performed at the 5th intercostal space. The pale yellow demarcated tumor was found to have invaded the chest wall involving the 1st to the 4th ribs, but the tumor did not adhere to the lung. Chest wall resection including the soft tissue tissues around the tumor was performed with adequate margins which were >2 cm away from the tumor. The chest wall defect measured 14 cm × 10 cm and was not reconstructed.
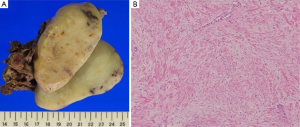
The tumor measured 9 cm × 6 cm × 6 cm, and a cross section showed it to be yellowish white in color (Figure 3A). Histopathologically, the tumor consisted of a proliferation of well-differentiated fibroblasts with collagenous bundles (Figure 3B). The cells exhibited sparse mitotic activity and the MIB-1 index was <5%. There were no cytological features of malignancy and an absence of necrosis. The tumor had invaded the periosteum and the intercostal muscle. The surgical margin showed no tumorous lesion. The final diagnosis was an intrathoracic desmoid tumor, and the patient was discharged uneventfully on postoperative day 13. She was in good health with neither recurrent signs of lung cancer nor desmoid tumor at 5 years after the second surgery.
Discussion
Desmoid tumors are clinicopathologically classified into three types: the extra-abdominal type; the abdominal type arising from musculoaponeurotic structures of the abdominal wall; and the intra-abdominal type arising in the pelvis or mesentery. Intrathoracic desmoid tumors contained in extra-abdominal desmoids grow invasively and insidiously, and cause little or no pain (4). Desmoid tumors do not usually metastasize, but slowly and locally advance. Therefore, the mainstay for the treatment is enblock surgical resection. However, the recurrence rates for desmoid tumors after excision are high and are directly related to the status of the surgical margin. Abbas et al. reported that 89% of patients with a positive surgical margin had recurrences, whereas only 18% with a negative resection margin had relapses (1). Furthermore, the diagnosis of a safe surgical margin is difficult using frozen sections. Consequently, radical resection with incision lengths as long as 2-4 cm is to be recommended (1,4).
There is a close relationship between familial adenomatous polyposis and Gardner’s syndrome, suggesting the role of an intrinsic genetic defect in the development of desmoid tumors. Estrogen has also been implicated in the multifactorial development of desmoid lesions because they tend to occur in women of reproductive age; some tumors express estrogen and progesterone receptors. Trauma or surgery can also be associated with the etiology. One patient in four with a desmoid tumor has been reported to have a history of trauma (5). Some studies have recently reported that thoracic desmoid tumors developed after thoracotomy or thoracoscopic surgery (2,3). Desmoid tumors can develop not only at the wound site but also at a distance from the port and thoracotomy sites. Postoperative chronic inflammation under the following scenario was suspected as representing the etiology of this intrathoracic desmoid tumor at a distance from the surgical incision. The first factor was irritation by thoracoscopic procedures. The second was irritation by the tip of the chest drainage tube positioned in the apical thoracic cavity. The third was stress caused by stretching of the intercostal space during the extraction procedure (2).
In conclusion, the presence of an intrathoracic desmoid tumor should be considered in patients who have undergone thoracoscopic surgery, even when the tumor arises at a distance from the port and thoracotomy sites.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Abbas AE, Deschamps C, Cassivi SD, et al. Chest-wall desmoid tumors: results of surgical intervention. Ann Thorac Surg 2004;78:1219-23. [PubMed]
- Mori T, Yamada T, Ohba Y, et al. A case of desmoid-type fibromatosis arising after thoracotomy for lung cancer with a review of the English and Japanese literature. Ann Thorac Cardiovasc Surg 2014;20 Suppl:465-9. [PubMed]
- Miwa K, Kubouchi Y, Wakahara M, et al. Desmoid tumor requiring differentiation from port-site relapse after surgery for lung cancer. Asian J Endosc Surg 2014;7:182-4. [PubMed]
- Allen PJ, Shriver CD. Desmoid tumors of the chest wall. Semin Thorac Cardiovasc Surg 1999;11:264-9. [PubMed]
- Enzinger FM, Shiraki M. Musculo-aponeurotic fibromatosis of the shoulder girdle (extra-abdominal desmoid). Analysis of thirty cases followed up for ten or more years. Cancer 1967;20:1131-40. [PubMed]


