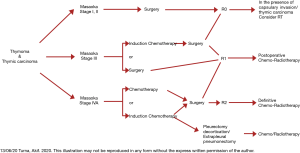
Multimodality approach in treatment of thymic tumors
As it has been well described in the literature, complete (i.e., R0) resection is of great importance in the treatment of thymic tumors (1). Thymomas are usually well capsulated and resectable without a need for resection of adjacent structure. However, extended thymectomy is advised due to the possibility of thymoma foci in the anterior mediastinal compartment (1). Other than stage IVB tumors (i.e., tumors with deep region lymph node metastasis or distant metastases including pulmonary metastases) tumors are considered resectable.
The therapeutic role of debulking surgery or subtotal/incomplete resection in stage III and IV disease has been controversial (1,2). It has been shown that, 5-year survival rate varies between 60% to 75% after incomplete resection and 24% to 40% after incisional biopsy alone (2-4). Radiotherapy after incomplete resection of thymic tumors provides local control rates of 35% to 74% and 5-year survival rates of 50% to 70% for stage III, 20% to 50% stage IVA tumors (4,5). Ciernik et al. reported that, tumor debulking did not improve prognosis when followed by radiation (6). They reported that stage III patients had a 5- and 10-year rates of 61% and 57% respectively, whereas 5- and 10-year survival rates of patients with stage IV disease 23% and 8%, respectively (6).
In essence, multidisciplinary approach to thymic tumors aims to improve the resectability and survival.
Multidisciplinary approach to thymic tumors has three forms:
- Induction chemotherapy implemented before surgery to shrink the bulk of inoperable stage IVA tumor, to reduce local involvement to mediastinal structures such as aorta, main pulmonary artery, atrium and potentially favoring downstaging; it is expected to allow a higher rate of complete resection.
- Induction/neoadjuvant chemotherapy administered before potentially operable stage III thymic tumor to increase the likelihood of complete resectional surgery.
- Adjuvant chemo-radiotherapy after surgery in patients who had histologically margin-free complete or incomplete resection in order to have lower local and distant recurrence after surgery.
Stage III thymic tumors demonstrate tumoral involvement into adjacent structures such as phrenic nerve, pulmonary parenchyma, great vessels such as brachiocephalic vein and superior vena cava and pericardium. In patients with Stage IVA tumors, pleural or pericardial tumoral implants or large tumors with non-discrete tumoral invasion can be seen (Figure 1). In these tumors, transthoracic tru-cut biopsy or mediastinotomy under local anesthesia is recommended since the patient can not be extubated due to extensive tracheal compression if a standard mediastinotomy is performed. Despite the fact that, most Stage III tumors can be resected with adjacent structures in the concept of ‘extended thymectomy’, induction therapies may increase the likelihood of complete resection (5,7-12). However, some of the patients with stage III tumors can be deemed as inoperable due to the extension and/or localization of the tumor.

Management of unresectable/marginally resectable stage III-IVA Thymic Tumors
For the stage III-IVA tumors, the standard approach should be obtained by a multidisciplinary team including thoracic surgeon, radiologist, pathologist, medical oncologist, radiation oncologist. In institutions where there is no multidisciplinary team, virtual tumor board with the help of online communication tools is recommended (The International Thymic Malignancy Interest Group. https://www.itmig.org). In patients who were deemed not to be a margin-negative surgical resection candidate, an induction chemotherapy can be applied (1,5,7-12).
For patients with locally advanced thymic tumor a complete radiologic staging that include a thin-sliced chest CT with contrast and PET-CT should be performed. Chest MRI is not recommended unless there is a vertebral invasion since MRI may exaggerate the invasion to great vessels and it may indicate inoperability in operable patients. In all patients with locally advanced thymic tumor a tissue diagnosis using tru-cut biopsy or mediastinotomy or extended mediastinoscopy should be obtained (13). A very widely mentioned dogma appoints a biopsy of possible thymic tumor out of regard of seeding in parietal pleura or biopsy site (14). No patient of seeding of a needle tract or biopsy area has never been reported. On the contrary, a trend to better survival who had undergone a preoperative biopsy was reported (15).
If a surgical margin-negative (R0) is deemed to be possible, tumor can resected by sternotomy with additional resection of pericardium, superior vena cava, innominate vein (Figures 2,3). There is a strong need for appropriate communication with pathologist in order to evaluate the resected specimen properly. Surgeon should show to pathologist the 4 horns of the tumor, pericardial and venous (vena cava superior or left/right innominate vein) surgical margins in detail. For locally advance tumor with adjacent tissue invasion, postoperative radiation treatment (PORT) is recommended if resection margins are close or positive. It has been shown that, especially the patients with thymic carcinomas may benefit from PORT (16).
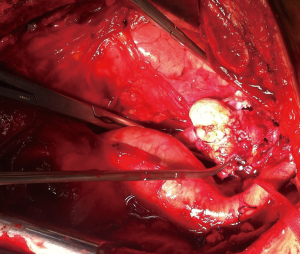
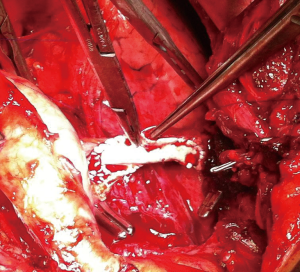
Thymomas are considered to be chemosensitive tumors and a variety of combinations of chemotherapy (Figure 4).
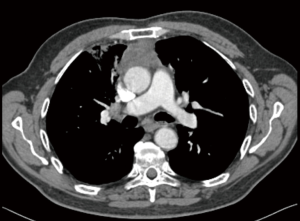
In the published studies regarding induction therapy of thymoma, clinical response rate varied between 62% to 100% and complete resection rates of 22% to 92% (Table 1). However, it should be emphasized that, there is no high-level evidence presenting randomized trial. The used regimen usually included cisplatin, cyclophosphamide and cyclophosphamide (CAP).

Full table
The effectiveness of chemotherapy has been validated in inoperable tumors (i.e., tumors invading main pulmonary artery, aorta, atrium etc.). Combination regimes generally have provided higher response rates higher response rates (21).
In one study, authors reported 22 patients with locally advanced inoperable tumor received neoadjuvant chemotherapy with CAP and prednisone followed by thymectomy, post operative radiotherapy and consolidation chemotherapy (23). The tumor was responded in 17 patients (72.7%) radiologically, 16 patients received surgical resection and 6 out of 16 patients (37.5%) showed marked (i.e., >80%) necrosis. In another study, the radiological response rate and complete resection rate was 73% and 77% respectively (24). Resectable patients underwent PORT.
Similar to other malignancies, the addition of radiation to induction chemotherapy has been administered in unresectable stage III-IVA thymic tumors. Loehrer and colleagues reported that (28), patients with unresectable thymoma and thymic carcinoma were given radiation at a dose of 54 Gy in addition to four cycles of cyclophosphamine, doxorubicin and cisplatin. In this study, no patient had progression, one out of 23 patients (4.3%) showed complete response, 16 patients (69.6%) demonstrated partial response (10). In another study (28), 8 out of 10 patients (80%) could have complete resection after 40–45 Gy of radiation therapy and chemotherapy consisting cisplatin/etoposide. In this series, 50% of patients (4 patients out of 8) were found have pathologic complete response. These studies provided has led to a strategy in patients thought to be unresectable or marginally-resectable resectable. Similarly, in a multi-institutional phase II study (29), 22 patients with stage III thymoma or thymic carcinoma were included if the tumor diameter was greater than 8 or 5 cm with irregular borders/ectopic calcification or vascular invasion. Ten out of 21 patients who completed the induction chemoradiotherapy (47%) showed radiological response. However, nine patients developed Grade 3 or 4 toxicity. In this series more than 90% of tumor were found to be responded to induction chemoradiotherapy (29). Overall survival was 71% at years, whereas 5-year progression free survival rate was found to be 83%. In this series, perioperative mortality was 9%.
Although, chemoradiotherapy provided greater response and complete necrosis, it can lead to greater toxicity before surgery as well as a greater postoperative complications. However, there is obviously no clear consensus on the best treatment modality for a thymic tumor that is deemed to unresectable because there is no high-level evidence. Table 2 shows the results of surgical resection of stage 4 thymomas.
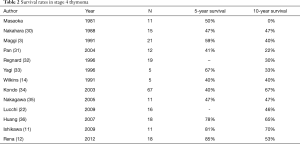
Full table
Induction radiation therapy
Radiation therapy applied as a sole therapeutical induction therapy modality has been rarely reported in the literature. Yagi and colleagues reported using Cobalt-based irradiation that, out of 30 patients with thymoma, 8 were given radiotherapy, and the survival rate of patients who received complete resection was higher than that of patients who had upfront resection (33). However, overall survival was not different between two groups. In a more recent series, authors could able to achieve complete resection in 10 of 19 patients who had preoperative radiation for invasion of vena cava and/or main pulmonary artery (37). Ahmad et al. reported that, only 6% of 1,042 patients from many institutions received induction radiotherapy alone before surgery (38).
Adjuvant chemotherapy
There is not enough data on the use of adjuvant therapy in thymic tumors after surgery. It has been reported that adjuvant chemotherapy did not increase survival rate in completely resected stage III and stage IV thymic tumors (34). There seems that there is no sufficient evidence to support for implementation of adjuvant chemotherapy. Recently, Kim and colleagues analyzed a large nation-wide cancer database of thymic carcinoma and they found that, adjuvant chemotherapy or radiotherapy was not associated with benefit in patients with stage IIB cancer who had complete resection of tumor (39). However, adjuvant therapy should be considered for stage IIB thymic carcinoma patients with positive margins and all patients with stage III thymic carcinoma (39).
Adjuvant radiation therapy
There is no sufficient evidence to recommend radiation therapy after stage I and stage II thymoma. However, it has been shown that radiation therapy following complete resection of stage III thymoma can reduce the incidence of recurrence in the tumor-adjacent areas (1). Gao and associates investigated the role of PORT in 188 patients with resected type B3 thymoma and concluded that adjuvant radiotherapy might be beneficial in stage III and IV patients (40). Adjuvant radiation therapy can be offered in more indolent tumors such as type B3 thymoma and later stages.
PORT in thymic tumors
For stage I thymoma, evidence suggest no beneficial role of PORT in terms of recurrence or 5-year survival (41,42). Similarly, Korst and colleagues performed a meta-analysis and systematic review (43) of retrospective series and they concluded that, there is no significant evidence to support PORT in patients with completely resected stage II and stage III thymomas. Mangi et al. also reported no beneficial impact of PORT in 45 patients with stage III thymoma (44). On the other hand, a retrospective analysis of SEER database revealed that, PORT improved survival in patients with stage III thymoma (45). Omasa and colleagues analyzed the Japanese Association for Research on the Thymus (JART) database and they concluded that, PORT improved recurrence-free survival in stage II and stage III thymic carcinomas but not in thymomas (46). On the other hand, analysis of Rimner and colleagues conducted an ITMIG database analysis and they found that, PORT increased overall survival in completely resected stage II and stage III thymomas (47). However, there is not much debate regarding the role of PORT in resected thymic carcinoma. Analysis of international (ITMIG) and Japanese (JART) database clearly indicates that, PORT is recommended in patients with thymic carcinoma. The large analysis of 2030 European Society of Thoracic Surgeons (ESTS) database done by Ruffini and colleagues revealed that, adjuvant treatment included radiation only or chemoradiotherapy increased survival of patients with completely resected stage II or stage III thymoma, neuroendocrine thymomas and thymic carcinoma (48).
As conclusion, postoperative radiotherapy can be recommended in patients with completely resected thymic carcinoma although, its role in other thymic tumors needs further investigation. Surveillance with radiation should be reserved for recurrences that are not amenable to re-resection. It should be kept in mind that, long-term follow-up is mandatory due to the indolent nature of these tumors.
Adjuvant chemoradiotherapy
Carillo and colleagues reported the results of postoperative chemoradiotherapy in 59 patients with completely resected stage II thymic tumors (49). They reported 92% of 10-year recurrence free survival. Despite the fact that, the study is an analysis of a retrospective series, the reported high survival rate is noteworthy.
Molecularly targeted therapy
Genetic profiles of thymic malignancies vary. As a targetable molecule epithelial growth factor receptor (EGFR) frequently is overexpressed in thymomas but activating mutations were reported to be rare (50). On the other hand, KIT mutations were found to be far less frequent (7% of thymic carcinomas) and are not correlated with KIT expression (51). Hu and colleagues reported as an analysis of 347 papers regarding targeted therapy against thymic malignancies that, c-KIT mutant thymic carcinomas could respond to target therapies (52). They also concluded that, the antiangiogenesis agent belinostat showed mild anti-tumour activity in heavily pretreated thymoma, but thymic carcinomas did not respond (51). It also seems that mTOR inhibitors as well as somatostatin analogues can be effective in some patients (53). Ongoing studies are assessing the opportunity of targeting emerging targets, including PI3K, CDK, and immune checkpoint PD-1/PD-L1.
Conclusions
Upfront surgical resection is recommended and provides high survival rate in Masaoka Stage 1 and 2 thymic tumors (Figure 5). However, a neoadjuvant therapy could be beneficial for stage 3 thymic tumors. Adjuvant therapy or additional chemo/radiotherapy should be tailored according to patient’s clinical status, stage of tumor and completeness of the resection (Figure 5). Evidence is scarce and varies for Stage IV tumors However, large randomized studies are warranted in order to clearly identify the best therapeutic options for thymic tumors.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Dragana Jovanovic and Semra Bilaceroglu) for the series “ Thymoma” published in Journal of Thoracic Disease. The article was sent for external peer review organized by the Guest Editors and the editorial office.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at: http://dx.doi.org/10.21037/jtd-20-818). The series “Thymoma” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ahmad U, Huang J. Tumors of the Thymus. In: Locicero J, Feins RH, Colson YL, et al. editor. Shields’ General Thoracic Surgery. 8th edition. Philadelphia: Wolters Kluwer, 2019:2144-60.
- Hamaji M, Kojima F, Omasa M, Sozu T, et al. A meta-analysis of debulking surgery versus surgical biopsy for unresectable thymoma. Eur J Cardiothorac Surg 2015;47:602-7. [Crossref] [PubMed]
- Maggi G, Casadio C, Cavallo A, et al. Thymoma: results of 241 operated cases. Ann Thorac Surg 1991;51:152-6. [Crossref] [PubMed]
- Cowen D, Mornex RF, Bachelot T, et al. Thymoma: results of multicentric retrospective series of 149 non-metastatic irradiated patients and review of the literature. Radiother Oncol 1995;34:9-16. [Crossref] [PubMed]
- Yokoi K, Matsuguma H, Nakahara R, et al. Multidisciplinary treatment for advanced invasive thymoma with cisplatin, doxorubicin, and methylprednisolone. J Thorac Oncol 2007;2:73-8. [Crossref] [PubMed]
- Ciernik IF, Meier U, Lütolf UM. Prognostic factors and outcome of incompletely resected invasive thymoma following radiationtherapy. J Clin Oncol 1994;12:1484-90. [Crossref] [PubMed]
- Bretti S, Berruti A, Loddo C, et al. Piemonte Oncology Network. Multimodal management of stages III-IVa malignant thymoma. Lung Cancer 2004;44:69-77. [Crossref] [PubMed]
- Yamada Y, Yoshino I, Nakajima J, et al. Surgical outcomes of patients with stage III thymoma in the Japanese Nationwide Database. Ann Thorac Surg 2015;100:961-7. [Crossref] [PubMed]
- Leuzzi G, Rocco G, Ruffini E, et al. Multimodality therapy for locally advanced thymomas: a propensity score-matched cohort study from the European Society of Thoracic Surgeons Database. J Thorac Cardiovasc Surg 2016;151:47-57. [Crossref] [PubMed]
- Wright CD, Choi NC, Wain JC, et al. Induction chemoradiotherapy followed by resection for locally advanced Masaoka stage III and IVA thymic tumors. Ann Thorac Surg 2008;85:385-9. [Crossref] [PubMed]
- Ishikawa Y, Matsuguma H, Nakahara R, et al. Multimodality therapy for patients with invasive thymoma disseminated into the pleural cavity: the potential role of extrapleural pneumonectomy. Ann Thorac Surg 2009;88:952-7. [Crossref] [PubMed]
- Rena O, Mineo TC, Casadio C. Multimodal treatment for stage IVA thymoma: a proposable strategy. Lung Cancer 2012;76:89-92. [Crossref] [PubMed]
- Metin M, Sayar A, Turna A, et al. Extended cervical mediastinoscopy in the diagnosis of anterior mediastinal masses. Ann Thorac Surg 2002;73:250-2. [Crossref] [PubMed]
- Wilkins EW, Grillo HC, Scannell JG, et al. Role of staging in prognosis and management of thymoma. Ann Thorac Surg 1991;51:888-92. [Crossref] [PubMed]
- Wilkins KB, Sheikh E, Green R, et al. Clinical and pathologic predictors of survival in patients with thymoma. Ann Surg 1999;230:562-72; discussion 572-4. [Crossref] [PubMed]
- Dong C, Chang S, Tang H. Role of Postoperative Radiation Therapy in Completely Resected Thymoma. Ann Thorac Surg 2017;103:364-5. [Crossref] [PubMed]
- Macchiarini P, Chella A, Ducci F, et al. Neoadjuvant chemotherapy, surgery, and postoperative radiation therapy for invasive thymoma. Cancer 1991;68:706-13. [Crossref] [PubMed]
- Rea F, Sartori F, Loy M, et al. Chemotherapy and operation for invasive thymoma. J Thorac Cardiovasc Surg 1993;106:543-9. [Crossref] [PubMed]
- Berruti A, Borasio P, Roncari A, et al. Neoadjuvant chemotherapy with adriamycin, cisplatin, vincristine and cyclophosphamide (ADOC) in invasive thymomas: results in six patients. Ann Oncol 1993;4:429-31. [Crossref] [PubMed]
- Venuta F, Rendina EA, Pescarmona EO, et al. Multimodality treatment of thymoma: a prospective study. Ann Thorac Surg 1997;64:1585-91. [Crossref] [PubMed]
- Kim ES, Putnam JB, Komaki R, et al. Phase II study of a multidisciplinary approach with induction chemotherapy, followed by surgical resection, radiation therapy, and consolidation chemotherapy for unresectable malignant thymomas: final report. Lung Cancer 2004;44:369-79. [Crossref] [PubMed]
- Lucchi M, Davini F, Ricciardi R, et al. Management of pleural recurrence after curative resection of thymoma. J Thorac Cardiovasc Surg 2009;137:1185-9. [Crossref] [PubMed]
- Kunitoh H, Tamura T, Shibata T, et al. A phase II trial of dose-dense chemotherapy, followed by surgical resection and/or thoracic radiotherapy, in locally advanced thymoma: report of a Japan Clinical Oncology Group trial (JCOG 9606). Br J Cancer 2010;103:6-11. [Crossref] [PubMed]
- Kawasaki H, Taira N, Ichi T, et al. Weekly chemotherapy with cisplatin, vincristine, doxorubicin, and etoposide followed by surgery for thymic carcinoma. Eur J Surg Oncol 2014;40:1151-5. [Crossref] [PubMed]
- Yue J, Gu Z, Yu Z, et al. Pretreatment biopsy for histological diagnosis and induction therapy in thymic tumors. J Thorac Dis 2016;8:656-64. [Crossref] [PubMed]
- Nakamura S, Kawaguchi K, Fukui T, et al. Multimodality therapy for thymoma patients with pleural dissemination. Gen Thorac Cardiovasc Surg 2019;67:524-9. [Crossref] [PubMed]
- Ma WL, Lin CC, Hsu FM, et al. Clinical Outcomes of Up-front Surgery Versus Surgery After Induction Chemotherapy for Thymoma and Thymic Carcinoma: A Retrospective Study. Clin Lung Cancer 2019;20:e609-18. [Crossref] [PubMed]
- Loehrer PJ Sr, Chen M, Kim K, et al. Cisplatin, doxorubicin, and cyclophosphamide plus thoracic radiation therapy for limited-stage unresectable thymoma: an intergroup trial. J Clin Oncol 1997;15:3093-9. [Crossref] [PubMed]
- Korst RJ, Bezjak A, Blackmon S, et al. Neoadjuvant chemoradiotherapy for locally advanced thymic tumors: a phase II, multi-institutional clinical trial. J Thorac Cardiovasc Surg 2014;147:36-44. [Crossref] [PubMed]
- Nakahara K, Ohno K, Hashimoto J, et al. Thymoma: results with complete resection and adjuvant post-operative irradiation in 141 consecutive patients. J Thorac Cardiovasc Surg 1988;95:1041-7. [Crossref] [PubMed]
- Pan CC, Wun HP, Yang CF, et al. The clinicopathological correlation of epithelial subtyping in thymoma: a study of 112 consecutive cases. Hum Pathol 1994;25:893-9. [Crossref] [PubMed]
- Regnard JF, Magdeleinat P, Dromer C, et al. Prognostic factors and long-term results after thymoma resection: a series of 307 patients. J Thorac Cardiovasc Surg 1996;112:376-84. [Crossref] [PubMed]
- Yagi K, Hirata T, Fukuse T, et al. Surgical treatment for invasive thymoma, especially when the superior vena cava is invaded. Ann Thorac Surg 1996;61:521-4. [Crossref] [PubMed]
- Kondo K, Monden Y. Therapy for thymic epithelial tumors: a clinical study of 1,320 patients from Japan. Ann Thorac Surg 2003;76:878-84. [Crossref] [PubMed]
- Nakagawa K, Matsuno Y, Kumitoh H, et al. Immunohistochemical KIT (CD117) expression in thymic epithelial tumors. Chest 2005;128:140-4. [Crossref] [PubMed]
- Huang J, Rizk NP, Travis WD, et al. Feasibility of multimodality therapy including extended resections in stage IVA thymoma. J Thorac Cardiovasc Surg 2007;134:1477-83; discussion 1483-4. [Crossref] [PubMed]
- Ribet M, Voisin C, Gosselin B, et al. Lympho-epithelial thymoma. Anatomo-clinical and therapeutic study of 113 cases. Rev Mal Respir 1988;5:53-60. [PubMed]
- Ahmad U, Yao X, Detterbeck F, et al. Thymic carcinoma outcomes and prognosis: results of an international analysis. J Thorac Cardiovasc Surg 2015;149:95-100. [Crossref] [PubMed]
- Kim S, Bull DA, Hsu CH, et al. The role of adjuvant therapy in advanced thymic carcinoma: A national cancer database analysis. Ann Thorac Surg 2020;109:1095-103. [Crossref] [PubMed]
- Gao L, Wang C, Fang W, et al. Outcome of Multimodality Treatment for 188 Cases of Type B3 Thymoma. J Thorac Oncol 2013;8:1329-34. [Crossref] [PubMed]
- Singhal S, Shrager JB, Rosenthal DI, et al. Comparison of stages I-II thymoma treated by complete resection with or without adjuvant radiation. Ann Thorac Surg 2003;76:1635-41. [Crossref] [PubMed]
- Utsumi T, Shiono H, Kadota Y, et al. Postoperative radiation therapy after complete resection of thymoma has little impact on survival. Cancer 2009;115:5413-20. [Crossref] [PubMed]
- Korst RJ, Kansler AL, Christos PJ, et al. Adjuvant radiotherapy for thymic epithelial tumors: a systematic review and meta-analysis. Ann Thorac Surg 2009;87:1641-7. [Crossref] [PubMed]
- Mangi AA, Wain JC, Donahue DM, et al. Adjuvant radiation of stage III thymoma: is it necessary? Ann Thorac Surg 2005;79:1834-9. [Crossref] [PubMed]
- Weksler B, Shende M, Nason KS, et al. The role of adjuvant radiation therapy for resected stage III thymoma: a population-based study. Ann Thorac Surg 2012;93:1822-8. [Crossref] [PubMed]
- Omasa M, Date H, Sozu T, et al. Japanese Association for Research on the Thymus. Postoperative radiotherapy is effective for thymic carcinoma but not for thymoma in stage II and III thymic epithelial tumors: the Japanese Association for Research on the Thymus Database Study. Cancer 2015;121:1008-16. [Crossref] [PubMed]
- Rimner A, Yao X, Huang J, et al. Postoperative radiation therapy is associated with longer overall survival in completely resected stage II and III thymoma-an analysis of the international thymic malignancies interest group retrospective database. J Thorac Oncol 2016;11:1785-92. [Crossref] [PubMed]
- Ruffini E, Detterbeck F, Van Raemdonck D, et al. European Association of Thoracic Surgeons (ESTS) Thymic Working Group. Tumours of the thymus: a cohort study of prognostic factors from the European Society of Thoracic Surgeons database. Eur J Cardiothorac Surg 2014;46:361-8. [Crossref] [PubMed]
- Carillo C, Diso D, Mantovani S, et al. Multimodality treatment of stage II thymic tumors. J Thorac Dis 2017;9:2369-74. [Crossref] [PubMed]
- Girard N, Shen R, Guo T, et al. Comprehensive genomic analysis reveals clinically relevant molecular distinctions between thymic carcinomas and thymomas. Clin Cancer Res 2009;15:6790-9. [Crossref] [PubMed]
- Girard N. Thymic tumors: relevant molecular data in the clinic. J Thorac Oncol 2010;5:S291-5. [Crossref] [PubMed]
- Hu B, Rong H, Han Y, et al. Do thymic malignancies respond to target therapies? Interact Cardiovasc Thorac Surg 2015;20:855-9. [Crossref] [PubMed]
- Merveilleux du Vignaux C, Maury JM, et al. Novel Agents in the Treatment of Thymic Malignancies. Curr Treat Options Oncol 2017;18:52. [Crossref] [PubMed]