Higher expression of SIRT1 induced resistance of esophageal squamous cell carcinoma cells to cisplatin
Introduction
Esophageal cancer is a kind of malignant digestive tract tumor with a potent invasiveness (1). Currently, surgical resection is a common therapeutic strategy for early-stage esophageal cancer. However, with the progression of the esophageal cancer, the recurrence rate after surgery increases gradually, and the outcome is not satisfactory (2). Therefore, for advanced or recrudescent esophageal cancer patients, chemotherapy combined with surgery is essential. However, the clinical effect of chemotherapy targeted with esophageal cancer is not satisfactory. Due to individual difference, the sensitivity of patients to chemotherapy varies. Low sensitivity even chemo-resistance would lead to a poor outcome along with elevating economic burden to patients. Esophageal cancer chemo-resistance to cisplatin, a widely used chemotherapeutic agent nowadays, has already been reported (3). Yet, the underlying mechanism of reduced sensitivity of cisplatin is not clear.
Sirtuin type 1 (SIRT1), a kind of nicotinamide adenine dinucleotide (NAD+)—dependent histone deacetylase, is one of the seven members of the sirtuin family. SIRT1 involves in DNA damage repair, cell cycle, apoptosis and oxidative stress in normal cells. While for tumor cells, SIRT1 has an anti-apoptotic effect and thus promoting carcinogenesis (4). Overexpression of SIRT1 has been found in a variety of solid tumors (5). Recently, some studies focus on the relationship between SIRT1 expression and efficacy of chemotherapy. Studies have shown that, possible mechanisms of SIRT1 and chemo-resistance involve Mdr-1, P-pg, FOXO3 and other signaling pathways (6).
Noxa is one member of the Bcl-2 family with pro-apoptotic effect, has been found overexpressed in 3,4,5,4’-tetramethoxystilbene (DMU-212)-treated colon cancer cells (7). Another study showed that Noxa might be related with sensitivity of Bcl-2 inhibitor ABT-737 in small cell lung cancer, as low Noxa expression could inhibit the apoptotic effect of ABT-737 (8).
Previous study has shown that high SIRT 1 expression had a significantly higher chance to be resistant to platinum-based chemotherapy (9). Mutation or expression changes of P53 induced cisplatin resistance and SIRT1 can regulate the deacetylation P53 and change its activation (10,11). As a downstream gene of P53, we suspect that Noxa may be also involved with cisplatin resistance and be regulated by SIRT1. However, whether SIRT 1 and Noxa play a role in cisplatin resistance of esophagus cancer should be analyzed.
Materials and methods
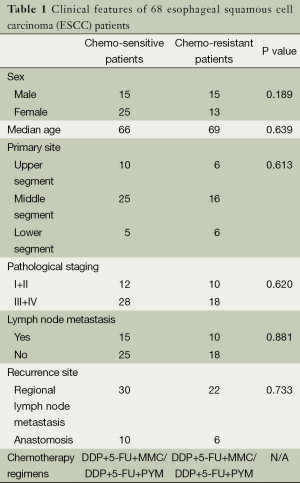
Sixty-eight patients (30 men and 38 women with the median age of 67 years) with histopathologically proven ESCC were included in this study, the clinical characteristics of patients was shown in Table 1. All patients underwent surgical treatment but developed recurrence. In order to control the recurrence, the patients received cisplatin based combination chemotherapy between May 2012 and February 2014 in Nanjing Drum Tower Hospital, China. To evaluate the effect of chemotherapy, we followed the RECIST (Response Evaluation Criteria in Solid Tumors) guideline. By calculating the diameter of tumor, patients were assessed as complete response (CR), partial response (PR), stable disease (SD) and progressive disease (PD). We defined CR + PR patients as chemo-sensitive patients and the others as chemo-resistant patients. In brief, 40 patients were assessed as chemo-sensitive patients while 28 patients were chemo-resistant. Excepting the curative effect of chemotherapy, the information about demographic data such as age, sex, stage of disease had no statistical difference between the chemo-sensitive group and the chemo-resistant group.
Full table
Cell lines
Human esophageal squamous cell carcinoma (ESCC) cell line (ECa9706 cells) was purchased from Beijing Zhongyuan Ltd (Beijing, China). ECa9706 cells were maintained in PRMI1640 medium (Thermo Fisher Scientific, USA) supplemented with 10% fatal bovine serum (Teaching Biological, Hangzhou, China) at 37 °C in the 5% CO2 incubator (Hera cell 150i, Thermo Fisher Scientific, USA). According to Wang’s methods (9), a cisplatin-resistant subline named ECa9706-CisR cell line was obtain from parental ECa9706 cells through a continuous exposure to increasing cisplatin (from 1.5 to 12 μM) over 12 months.
Quantitative real-time reverse PCR (qRT-PCR) for SIRT1 and Noxa mRNA expression
Total RNA extraction of tissues and cells were performed using Trozol reagent (Thermo Fisher Scientific, USA). Qualified RNA was used for cDNA synthesis with PrimeScript® RT reagent (Takara Biotechnology, Dalian, China) according to manufacturer’s instruction. cDNA was amplified with SYBR® Premix Ex Taq™ II kit(Takara Biotechnology, Dalian, China) on a 7900HT fast real-time PCR system (Applied Biosystems, USA). The cycling conditions were set by manufacturer’s protocol. Primers of targeted genes and β-actin were as follows: SIRT1, 5'GCC TCA TCT GCA TTT TGA TG'3(sense), 5'TCT GGC ATG TCC CAC TAT CA'3(antisense). Noxa, 5'TTC GTG TTC AGC TCG CGT CC’3(sense), 5'CTC GGT GTA GCC TTC TTG CC'3(antisense). β-actin, 5' CTC CAT CCT GGC CTC GCT GT'3(sense), 5'GCT GTC ACC TTC ACC GTT CC'3(antisense). Using ΔΔ cycle threshold (2-ΔΔCt) method determined the fold change of SIRT1 and expression data were normalized by β-actin.
Western blot analysis for SIRT1 and Noxa expression
Total protein in tissue samples, ECa9706 cells and ECa9706-CisR cells were extracted using the RIPA reagent containing 1% protease inhibitors cocktail (Applygen, Beijing, China). The protein concentration in sample was determined by BCA kit(Applygen, Beijing, China) and pre-treated with 6× Loading Buffer at 100 °C for 3-5 min. Protein 45 μg was separated on 10% SDS-polyacrylamide gel and then transferred polyvinylidene difluoride (PVDF) membranes. Blots were blocked with 5% skim milk in PBS for 1 h at room temperature and incubated at 4 °C for 12 h with the primary antibody anti-SIRT1 (1:500, Santa Cruz Biotechnology, USA), anti-Noxa (1:1,000, Santa Cruz Biotechnology, USA) and anti-β-actin (1:3,000, Santa Cruz Biotechnology, USA). Membranes were washed by PBST buffer and followed an incubation with horseradish peroxidase-conjugated secondary antibodies. ECL kit (Applygen, Beijing, China) was used to detected the band signals.
Transfection of siRNA targeted to SIRT1
Inhibition of SIRT1 expression in ECa9706-CisR cells was induced using specific siRNA(Sequence-sense: GCA AUA GGC CUC UUA AUU Att; antisense: UAA UUA AGG CCU AUU GCtt). Target siRNA and control siRNA were all designed and synthesized by Shanghai GenePharma Company (China). ECa9706-CisR cells were planted into 24 well-plate with a density of 1×105 and transfected with 60 nM siRNA using Lipofectamine® 2000 reagent (Life Technologies Corporation, USA) according manufacture’s protocol, while silencer negative control siRNA was used. To confirm the down-regulation effect of siRNA, qRT-PCR and western blot was utilized to determine the level of SIRT1 at 24 and 48 h respectively.
Cell proliferation analysis
CCK-8 assay was performed to analyze the resistance of ECa9706-CisR cells to cisplatin. Non-transfected or SIRT1 silenced ECa9706-CisR cells were planted into 96 well-plate with a density of 1×104/100 μL and treated with 5 μM cisplatin for 24 h. As control, non-transfected ECa9706-CisR cells were cultured without any treatment. After 24 h, cells were incubated with CCK-8 counting reagent for 4 h at 37 °C. The optical density (at 450 nm) in each well was measured by enzyme-linked immunosorbent assay plate reader and viability rate was calculated.
Apoptosis and cell cycle analysis
For cell apoptosis analysis, ECa9706-CisR cells were collected and Annexin V-FITC assay kit was used according to the manufacturer’s protocol after treated with 5 μM cisplatin for 24 h. For cell cycle analysis, treated cells was fixed in 70% cold ethanol and were stained with 50 μg/mL propidium iodide (Sigma-Aldrich, USA) and incubated for 30 min. Analysis was performed using a BD FACSCalibur flow cytometer (Beckman Coulter, USA) within 15 min. Using CellQuest software analyzed cell cycle and apoptosis rate.
Noxa expression analysis
Total mRNA and protein in different ECa9706-CisR cells were extracted after treated with 5 μM cisplatin for 24 h. The expression of Noxa after SIRT1 inhibition was determined using qRT-PCR and Western blot with the protocol described above.
Statistical analysis
Statistical analysis of the data took advantage of SPSS 13.0 statistical software (SPSS, Chicago, IL, USA). The results were expressed as mean ± SD. Date were compared between two groups or multiple groups using Student’s t-test and one-way analysis of variance respectively. Non-normal distribution data was analyzed by Mann-Whitney test. The P<0.05 was considered as statistically significant in this study.
Results
Expression of SIRT1 and Noxa in tumor tissue specimens
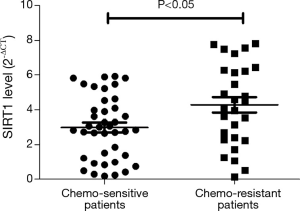
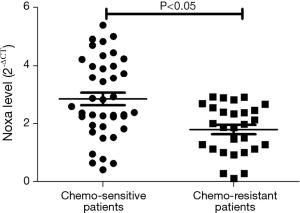
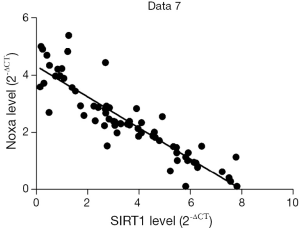
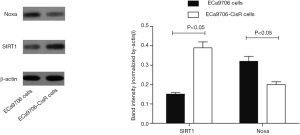
We evaluated the expression of SIRT1 in patients with ESCC. qRT-PCR analysis demonstrated higher expression of SIRT1 and lower expression of Noxa in chemo-resistant patients compared to chemo-sensitive patients (P=0.008 and 0.000, respectively) (shown in Figures 1,2). The western blot also confirmed the higher level of SIRT1 and lower level of Noxa in chemo-resistant patients (Figure 3). Correlation analysis showed a negative correlation between SIRT1 and Noxa in mRNA level (r=−0.803 P=0.000) (Figure 4), indicating that SIRT1 is associated with sensitivity of patients to chemotherapy and Noxa expression is also possibly involved.
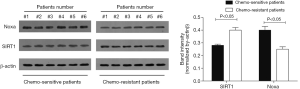
Expression of SIRT1and Noxa in ECa9706 cells and ECa9706-CisR cells
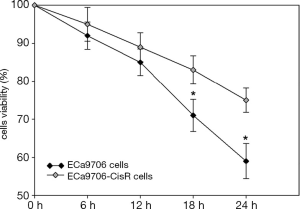
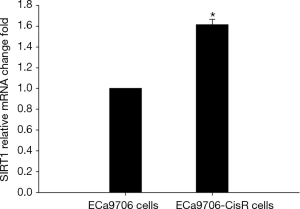
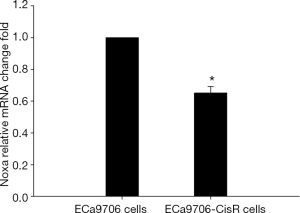
The resistance to cisplatin of ECa9706-CisR cells was proven in preliminary experiment (Figure 5). We evaluated the expression of SIRT1 and Noxa in ECa9706 cells and ECa9706-CisR cells. qRT-PCR analysis demonstrated higher level of mRNA and lower level of Noxa mRNA in ECa9706-CisR cell compared to the ECa9706 cells (P=0.000 and 0.003 respectively). The western blot also confirmed the results of qRT-PCR analysis (Figures 6-8).
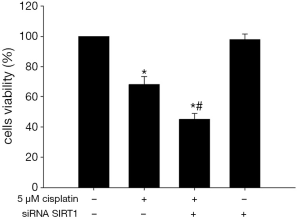
SIRT1 Inhibition improves the proliferation inhibitory effect of cisplatin
As expected, 5 μM cisplatin could inhibit proliferation of ECa9706-CisR cells significantly (Figure 9). After 5 μM cisplatin treatment, the viability rate of non-transfected ECa9706-CisR cells was (73.3±6.9)%. However, the viability rate of SIRT1 silenced ECa9706-CisR cells was further lower (55.4%±4.8%) and this difference was statistically significant (P=0.000).
SIRT1 inhibition enhances the apoptosis caused by cisplatin
As shown in Figure 10, flow cytometry demonstrated that SIRT1 inhibition enhances the apoptosis rate from (23.5±1.9)% in 5 μM cisplatin treated non-transfected ECa9706-CisR cells to (35.6±2.8)% in 5 μM cisplatin treated SIRT1 silenced ECa9706-CisR cells, and the difference between them was statistically significant (P=0.000).

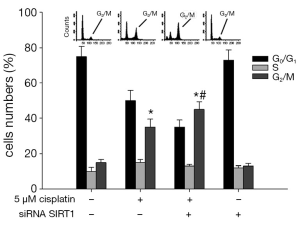
SIRT1 Inhibition aggravates the G2/M phase arrest induced by cisplatin
As shown in Figure 11, we tested the impact of SIRT1 inhibition on cell cycle arrest induced by cisplatin. In the control (non-transfected ECa9706-CisR cells without cisplatin treatment) the percentages of G0/G1 phase and G2/M phase were (70.3±4.1)% and (4.5±0.7)%, respectively. With the 5 μM cisplatin treatment, in control cells, the proportion of G0/G1 phase fell to (56.3±5.2)%, and the proportion of G2/M phase increased to (35.5±4.1)%, suggesting G2/M phase arrest occurred. Meanwhile, in SIRT1 silenced ECa9706-CisR cells, the proportion of G0/G1 phase was lower (35.5±6.5)% and the percentage of cells in the G2/M phase was further increased (45.3±5.9)%, compared with control and SIRT1 silenced ECa9706-CisR cells without cisplatin treatment.
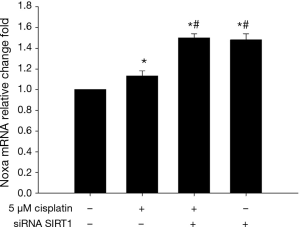
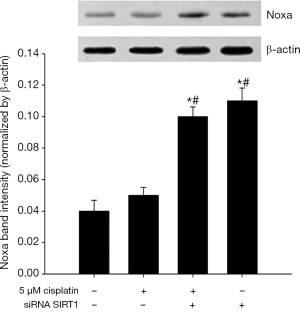
SIRT1 inhibition increases the expression of Noxa
As shown in Figures 12 and 13, 5 μM cisplatin induced slight higher expression of Noxa in ECa9706-CisR cells compared with control. More excitedly, obvious higher expression was observed after SIRT1 targeted siRNA transfection. It suggested that SIRT1 can improve sensitivity of ESCC cells to cisplatin by regulating Noxa.
Discussion
The present treatments of esophageal cancer are surgery, chemotherapy, radiotherapy and immunotherapy, among which surgery is the preferred strategy of early cancer (12). But for the advanced cancer patients, the effect of surgical treatment is less effective compared with the early cancer, adjuvant radiotherapy, and chemotherapy are always necessary for them (13,14). Especially in China, most of the patients with esophageal cancer are at the advanced stage when diagnosed with cancer, and lose the opportunity of surgery (15,16). For these patients, chemotherapy is very important. Cisplatin is an effective broad spectrum anticancer drug; however, extensively published studies have reported the cisplatin resistance in human cancer cells both in vivo and in vitro (17,18). We have to admit that there are complexities of cisplatin sensitivity and resistance. Changes can occur in almost every mechanism influencing cell growth, developmental pathways, apoptosis, DNA repair, drug metabolism, drug transporters (19). In this study, we focused on abnormal expression of SIRT1. High expression of SIRT1 existed in both chemo-resistant patients and ECa9706-CisR cells resistance to cisplatin. More importantly, after further using siRNA to silence SIRT1, ECa9706-CisR cells sensitivity to cisplatin improved, which showed cell viability decreased, G2/M arrest proportion increased, and apoptosis rate increased. Actually, previous studies have showed that SIRT1 may influence the sensitivity of tumor cells to chemotherapeutical agents, such as Chen et al. found that overexpression of SIRT1 promoted tumor genesis and resistance to chemotherapeutical agent and sorafenib (20). Kojima et al. found that up-regulation of SIRT1 expression may play an important role in promoting cell growth and chemo-resistance in androgen-refractory prostate cancer PC3 and DU145 cells (21).
In the aspect of tumor drug resistance, some studies found that after using RNA interfering technology to reduce SIRT1 expression, P-glycoprotein (P-gp) and multidrug resistance (MDR) proteins expression also decreased accordingly, and the latter two overexpression were the important reason for the tumor drug resistance (22,23). Transient transfection experiments showed that in human embryo kidney cells, SIRT1 can directly induce gene expression of MDR1 which resulted in the decrease of cellular sensitivity to drug (24). These studies indicated that SIRT1 inhibiting can partly improve sensitivity of tumor to chemotherapy drugs. Our studies found that, SIRT1 affected the resistance of cells to chemotherapeutical agents such as cisplatin by adjusting Noxa. Noxa’s effect of promoting apoptosis has been relatively clear, such as Baou et al. found that Noxa high expression played a key role in the process during which bortezomib inhibit chronic lymphocyte leukemia cells (25). It is generally acknowledged that Noxa play a role of promoting apoptosis mainly through mitochondrial cytochrome C-way, after combing with mitochondria, Noxa can affect mitochondrial permeability, the outer membrane potential, which lead to the release of cytochrome c, to activate Caspase family and induce apoptosis (26). Most importantly, Noxa expression is regulated by P53, P53 binding sites exist in its upstream startup sequence, when cells get damaged by chemotherapy drugs or oxidative stress, P53 will combine with Noxa startup sequence and up regulate its expression (27,28). Based on this, P53 might be a relational bridge between SIRT1 and Noxa. But the effect of promoting apoptosis by Noxa partly depend on the P53, in some cases, such as in hypoxia, Noxa can be activated through the non-P53 pathways, and other studies have shown that transcriptional factor E2F1 can raise Noxa directly (29,30). In this study, whether or not there are other pathways independent of P53 which can activate Noxa awaits further research.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Vaiphei K, Sinha SK, Kochhar R. Comparative analysis of Oct4 in different histological subtypes of esophageal squamous cell carcinomas in different clinical conditions. Asian Pac J Cancer Prev 2014;15:3519-24. [PubMed]
- Wang J, Ge J, Zhang XH, et al. Endoscopic submucosal dissection versus endoscopic mucosal resection for the treatment of early esophageal carcinoma: a meta-analysis. Asian Pac J Cancer Prev 2014;15:1803-6. [PubMed]
- Akutsu Y, Shuto K, Kono T, et al. A phase 1/11 study of second-line chemotherapy with fractionated docetaxel and nedaplatin for 5-FU/cisplatin-resistant esophageal squamous cell carcinoma. Hepatogastroenterology 2012;59:2095-8. [PubMed]
- Hwang BJ, Madabushi A, Jin J, et al. Histone/protein deacetylase SIRT1 is an anticancer therapeutic target. Am J Cancer Res 2014;4:211-21. [PubMed]
- Lv L, Shen Z, Zhang J, et al. Clinicopathological significance of SIRT1 expression in colorectal adenocarcinoma. Med Oncol 2014;31:965. [PubMed]
- Portmann S, Fahrner R, Lechleiter A, et al. Antitumor effect of SIRT1 inhibition in human HCC tumor models in vitro and in vivo. Mol Cancer Ther 2013;12:499-508. [PubMed]
- Piotrowska H, Myszkowski K, Amarowicz R, et al. Different susceptibility of colon cancer DLD-1 and LOVO cell lines to apoptosis induced by DMU-212, a synthetic resveratrol analogue. Toxicol In Vitro 2013;27:2127-34. [PubMed]
- Shiozaki H, Sudo K, Xiao L, et al. Distribution and timing of distant metastasis after local therapy in a large cohort of patients with esophageal and esophagogastric junction cancer. Oncology 2014;86:336-9. [PubMed]
- Zhang T, Rong N, Chen J, et al. SIRT1 expression is associated with the chemotherapy response and prognosis of patients with advanced NSCLC. PLoS One 2013;8:e79162. [PubMed]
- Chee JL, Saidin S, Lane DP, et al. Wild-type and mutant p53 mediate cisplatin resistance through interaction and inhibition of active caspase-9. Cell Cycle 2013;12:278-88. [PubMed]
- Lhee SJ, Song EK, Kim YR, et al. SIRT1 Inhibits p53 but not NF-κB Transcriptional Activity during Differentiation of Mouse Embryonic Stem Cells into Embryoid Bodies. Int J Stem Cells 2012;5:125-9. [PubMed]
- Wang TH, Wan JY, Gong X, et al. Tetrandrine enhances cytotoxicity of cisplatin in human drug-resistant esophageal squamous carcinoma cells by inhibition of multidrug resistance-associated protein 1. Oncol Rep 2012;28:1681-6. [PubMed]
- Kubota K, Kuroda J, Yoshida M, et al. Surgical therapy and chemoradiotherapy for postoperative recurrent esophageal cancer. Hepatogastroenterology 2013;60:1961-5. [PubMed]
- Mirinezhad SK, Somi MH, Shirmohamadi M, et al. Impact of postoperative chemoradiotherapy and chemoradiotherapy alone for esophageal cancer in North-West Iran. Asian Pac J Cancer Prev 2013;14:3921-4. [PubMed]
- Yuan P, Chen TH, Chen ZW, et al. Calculation of life-time death probability due malignant tumors based on a sampling survey area in China. Asian Pac J Cancer Prev 2014;15:4307-9. [PubMed]
- Zheng L, Tang W, Shi Y, et al. p21 rs3176352 G>C and p73 rs1801173 C>T polymorphisms are associated with an increased risk of esophageal cancer in a Chinese population. PLoS One 2014;9:e96958. [PubMed]
- Cheng Y, Li K, Diao D, et al. Expression of KIAA0101 protein is associated with poor survival of esophageal cancer patients and resistance to cisplatin treatment in vitro. Lab Invest 2013;93:1276-87. [PubMed]
- Ajani JA, Wang X, Song S, et al. ALDH-1 expression levels predict response or resistance to preoperative chemoradiation in resectable esophageal cancer patients. Mol Oncol 2014;8:142-9. [PubMed]
- Shen DW, Pouliot LM, Hall MD, et al. Cisplatin resistance: a cellular self-defense mechanism resulting from multiple epigenetic and genetic changes. Pharmacol Rev 2012;64:706-21. [PubMed]
- Chen HC, Jeng YM, Yuan RH, et al. SIRT1 promotes tumorigenesis and resistance to chemotherapy in hepatocellular carcinoma and its expression predicts poor prognosis. Ann Surg Oncol 2012;19:2011-9. [PubMed]
- Kojima K, Ohhashi R, Fujita Y, et al. A role for SIRT1 in cell growth and chemoresistance in prostate cancer PC3 and DU145 cells. Biochem Biophys Res Commun 2008;373:423-8. [PubMed]
- Chu F, Chou PM, Zheng X, et al. Control of multidrug resistance gene mdr1 and cancer resistance to chemotherapy by the longevity gene sirt1. Cancer Res 2005;65:10183-7. [PubMed]
- Zhu H, Xia L, Zhang Y, et al. Activating transcription factor 4 confers a multidrug resistance phenotype to gastric cancer cells through transactivation of SIRT1 expression. PLoS One 2012;7:e31431. [PubMed]
- Kaewpiboon C, Srisuttee R, Malilas W, et al. Extract of Bryophyllum laetivirens reverses etoposide resistance in human lung A549 cancer cells by downregulation of NF-κB. Oncol Rep 2014;31:161-8. [PubMed]
- Baou M, Kohlhaas SL, Butterworth M, et al. Role of NOXA and its ubiquitination in proteasome inhibitor-induced apoptosis in chronic lymphocytic leukemia cells. Haematologica 2010;95:1510-8. [PubMed]
- Pang X, Zhang J, Lopez H, et al. The carboxyl-terminal tail of Noxa protein regulates the stability of Noxa and Mcl-1. J Biol Chem 2014;289:17802-11. [PubMed]
- Hamard PJ, Barthelery N, Hogstad B, et al. The C terminus of p53 regulates gene expression by multiple mechanisms in a target- and tissue-specific manner in vivo. Genes Dev 2013;27:1868-85. [PubMed]
- Marshall AD, Picchione F, Geltink RI, et al. PAX3-FOXO1 induces up-regulation of Noxa sensitizing alveolar rhabdomyosarcoma cells to apoptosis. Neoplasia 2013;15:738-48. [PubMed]
- Kim JY, Ahn HJ, Ryu JH, et al. BH3-only protein Noxa is a mediator of hypoxic cell death induced by hypoxia-inducible factor 1alpha. J Exp Med 2004;199:113-24. [PubMed]
- Bertin-Ciftci J, Barré B, Le Pen J, et al. pRb/E2F-1-mediated caspase-dependent induction of Noxa amplifies the apoptotic effects of the Bcl-2/Bcl-xL inhibitor ABT-737. Cell Death Differ 2013;20:755-64. [PubMed]