
Preoperative evaluation of the segmental artery by three-dimensional image reconstruction vs. thin-section multi-detector computed tomography
Introduction
Lung cancer has become the leading malignant lung disease and the main cause of cancer death in China and other countries around the world (1). With the extensive use of spiral computed tomography (CT), early-stage lung cancer is being more frequently detected. Surgery is the recommended treatment for patients with early-stage non-small cell lung cancer (NSCLC) (2). As an alternative treatment to lobectomy, video-assisted segmentectomy is being gradually accepted by more surgeons to cure early-stage NSCLC because of its many advantages, which include less postoperative pain, shorter length of stay, and better reserve of lung function (3-6). However, video-assisted segmentectomy requires that thoracic surgeons understand the anatomy precisely, especially the intersegmental veins which mark the borders of segments. Meanwhile, the ability of conventional CT images to assess lung anatomy is limited. Therefore, it is important, especially to an inexperienced surgeon, to have a clear, preoperative assessment of the patient’s pulmonary anatomy.
In recent years, three-dimensional (3D) simulation technology has been widely used in lobectomy and segmentectomy, and many 3D image reconstruction software packages have been developed. All the software has the same goal: preoperatively display NSCLC lesions and anatomic structures of the lung for surgeons by use of two-dimensional (2D) CT images to improve the safety and accuracy of VATS segmentectomy (7). Numerous recent studies have proven the practicability of these different software packages (7,8). Recently, our medical team independently developed our own free and open-source 3D image reconstruction software (people can get it by contacting with corresponding author with email and we will respond with the download link and a guide). We named it “Exoview.” Exoview is free of installation and easy to use. Moreover, it allows operators to mark out small vessels manually in the 2D view and thus making the 3D image reconstruction more precise. This software has been used in our daily clinical work and has achieved some success. However, the quality of the pulmonary 3D model determined by the CT images’ quality is affected by many factors, including the CT value of the pulmonary vessels and the status of the patient’s breath hold. Therefore, this study aimed to evaluate the efficiency of Exoview for preoperatively evaluating the pulmonary artery (PA) in VATS segmentectomy by comparison with thin-section multi-detector computed tomography (MDCT).
We present the following article in accordance with the “The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement” reporting checklist (available at http://dx.doi.org/10.21037/jtd-20-1014).
Methods
Patients
A retrospective analysis was performed on the cases of 52 patients who accepted anatomical segmentectomy from May 2018 to May 2019 at The First Affiliated Hospital of Soochow University. The indications for pulmonary segmentectomy included the following: (I) size of lesion less than 2 cm in diameter on CT; (II) solid portion of ground-glass lesions less than 50% (9); (III) lymph node diameter less than 10 mm. Patients who had a second primary lesion in a different lung or who had undergone wedge resection or multi-lobe resection were excluded. The preoperative examination included lung function, electrocardiogram, echocardiography, brain magnetic resonance imaging (MRI), and lower extremity ultrasonography. Surgical outcomes and patients’ preoperative characteristics were collected from the HaiTai database (Nanjing, Jiangsu, China). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This retrospective study was approved by The First Affiliated Hospital of Soochow University (ID: 2020129). All patients gave informed written consent.
Preoperative CT and 3D reconstruction
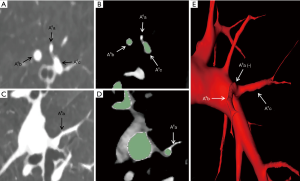
All patients underwent computed tomography pulmonary angiography (CTPA) by use of a 64-slice multi-detector CT (MDCT) unit (Aquilion-64, Toshiba Medical Systems, Tokyo, Japan) and injection of contrast medium (iodixanol, Visipaque 320, GE Healthcare, Cork, Ireland) preoperatively in order to distinguish the pulmonary arteries and veins. The contrast medium was injected by a dual-head power injector via the cubital vein, with 70–90 mL of the medium being injected at a rate of 5 mL/sec. The scanning from the apex to the costophrenic angles was initiated 4 sec after the injection started with patients’ breath-holding. The CT scan data were uploaded into the server of the Department of Radiology in Digital Imaging and Communication in Medicine (DICOM) format. We obtained the DICOM data at a slice thickness of 0.625 mm. A skilled thoracic surgeon (Jun Chen) extracted the pulmonary arteries from 2D data and then converted these data into a 3D format by use of Exoview and marked them out in red (Figure 1). The anatomic patterns of 3D images were evaluated by three experienced surgeons (Jun Zhao, Chang Li, and Chun Xu) who had prior experience of >500 pulmonary anatomical resections. The number and the origin of subsegmental arteries from which each targeted segments’ arteries branched were identified precisely using 3D images and thin-section MDCT images. Finally, the intraoperative findings of segmental arteries’ (SA) branches were compared with the 3D images and thin-section MDCT images in each patient’s case.
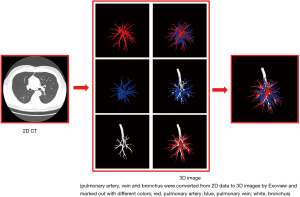
Operative procedure and anatomy
All operations were performed under general anesthesia and patients in the lateral decubitus position with lung exclusion by double-lumen intubation. A single-port or two-port anterior approach without rib spreading was prepared. A 2.5-cm incision was made at the fourth intercostal space along the anterior axillary line. In the two-port anterior approach, another 1 cm incision was made at the seventh intercostal space along the mid-axillary line. A 10 mm 30 degree thoracoscope (Karl Storz, Tuttlingen, Germany) was placed at the 1 cm incision. Harmonic scalpel (Ethicon Endo-Surgery Inc., Cincinnati, OH, USA) and electrocautery were used for routine energy devices. The bronchus, vessel, and fissure were managed with endoscopic staplers (Ethicon Endo-Surgery Inc., Cincinnati, OH, USA). After the bronchus was dissected, the anesthetist reinflated the lung on the operating side. A clear inflation-deflation line gradually appeared about 15–20 min after recollapsing the lung. Along the inflation-deflation line, the pulmonary parenchyma was dissected by endoscopic staplers. Mediastinal and lymph node sampling was performed. One chest tube was placed for postoperative drainage in all patients. Standard nomenclature and letter-number codes for pulmonary segments and arterial branches followed the method of Yamashita’s Roentgenologic Anatomy of the Lung (10).
Study parameters and statistical analysis
Study parameters included patient characteristics, operative factors, and the branching patterns of the PA of the targeted lung segments according to the intraoperative findings, 3D image reconstruction, and CT images. All statistical analyses were performed using SPSS 20.0 statistical software. Numerical data are expressed as the mean ± SD. Variables were compared using the two-sample t-test. The significance level was set as P<0.05.
Results
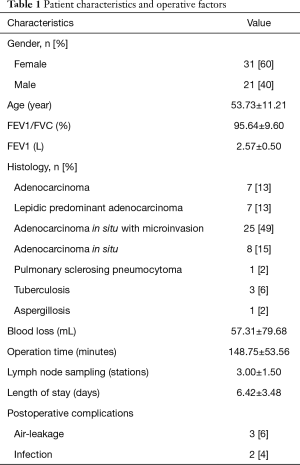
The patient characteristics and operative factors are listed in Table 1. The study cohort of 52 patients included 31 (60%) women and 21 (40%) men, who underwent segmentectomies. There were 7 accepted VATS lobectomies with radical lymph nodes dissection because invasive adenocarcinoma was confirmed by intraoperative frozen-section analysis, 1 in which mediastinal lymph node metastasis was detected by the postoperative pathologic result. There was 1 conversion from VATS segmentectomy to an open operation because of bleeding of the bronchial artery. The other operative factors of the 52 cases were as follows: operation time, mean of 148.75 min (±53.56); blood loss, mean of 57.31 mL (±79.68); postoperative hospitalization days, mean of 6.42 days (±3.48); lymph node sampling, mean of 3.00 stations (±1.50); postoperative complications, 5 (10%) patients. The pathologic results of 52 patients were adenocarcinoma in 7 patients, lepidic predominant adenocarcinoma in 7 patients, adenocarcinoma in situ in 8 patients, adenocarcinoma in situ with microinvasion in 25 patients, aspergillosis in 1 patient, pulmonary sclerosing pneumocytoma in 1 patient, and tuberculosis in 3 patients.
Full table
Tables 2 and 3 show the branching patterns of the PA of the targeted lung segment of the left and right lung respectively, according to the intraoperative findings. The right apical (n=10), left apicodorsal (n=8), right superior (n=8), right dorsal (n=7), and right ventral (n=6) segments were the most frequent segmentectomies performed. The other segmentectomies of the left lung included ventral (n=2), lingular (n=1), superior (n=4), ventrobasal (n=1), laterobasal (n=1), and upper division (n=2) segments. The other segmentectomies of the right lung included apicodorsal (n=1) and dorsobasal (n=1) segments.

Full table

Full table
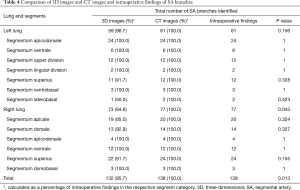
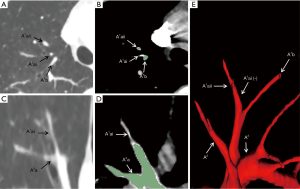
Basing on the intraoperative findings, a total of 138 SA branches were examined. A total of 95.7% (132 of 138) and 100% (138 of 138) of these SA branches were precisely identified on preoperative 3D image reconstruction and thin-section MDCT images, respectively (Table 4). The thin-section CT images’ ability to detect SA branches was significantly higher than that of 3D image reconstruction (P=0.013). On the left lung, 3D image reconstruction missed 1 SA branch of the superior segment and 1 of the laterobasal segment. On the right lung, 3D image reconstruction missed 1 branch of the apical segment, 1 branch of the dorsal segment, and 2 branches of the superior segment. These missed branches were <1.4 mm in diameter (Figures 2,3).
Full table
Discussion
We compared three-dimensional image reconstruction and thin-section MDCT images for preoperative evaluation of the SA branches of segmentectomy. Our results indicate that the thin-section MDCT images provide a better evaluation of smaller SA branches.
With the development of thoracic surgery, video-assisted thoracoscopic surgery (VATS) has become the routine approach for the management of lung cancer because of its many advantages, which include fewer complications, shorter length of stay, and improved pain control (9,11,12). In the process of anatomical segmentectomy, a shared visualization of the surgical field provided by VATS is useful because intraoperative discussion based on a shared view is as valuable as preoperative planning to prevent the misidentification of anatomical landmarks (13). With the advent of the 3D reconstruction technique, the pulmonary structures (vessels, bronchus, and nodules) can be rotated freely and visualized interactively from any angle. This “surgery map” enables surgeons to accurately manage pulmonary structures despite the presence of anatomical variation and improves the efficiency of endoscopic staplers, leading to an overall decrease in the financial burden to the patient (8).
Several similar studies have evaluated the use of different three-dimensional technologies in thoracic surgery. Watanabe and his colleagues used the 3D-CT pulmonary angiography (3D-CTPA) to successfully identify 98% (84 of 86) of PA branches in 14 patients. The 2 missed branches were less than 1.5 mm in diameter (14). Fukuhara et al. reported 95.2% (139 of 146) of PA branches were precisely identified on preoperative 3D-CT angiography, with the 7 missed branches being less than 2 mm in diameter (15). In Akiba et al.’s study, 27 patients underwent lobectomy or segmentectomy. They identified 95% arteries by 3D-CT, and the smallest PA diameter was 1.2 mm (16). Murota and colleagues evaluated PA branches of the right upper lobe by 3D-CTPA and thin-section multiplanar reconstruction (MPR) images. They concluded that 97.2% (316 of 325) and 99.7% (324 of 325) of PA branches of the right upper lobe were identified by 3D-CTPA and MPR images, respectively. The 9 vessels missed by 3D-CTPA were less than 1.4 mm in diameter (17).
In our study, the 3D reconstruction software “Exoview,” which was independently developed by our medical team, detected 95.7% (132 of 138) of the subsegmental arteries of 52 patients and the smallest branch was 1.4 mm in diameter. This result was consistent with previous studies. It appears that using 3D technologies in thoracic surgery has run into a bottleneck. However, with the higher morbidity of lung cancer and more early-stage lung cases being detected, sublobar resections, including anatomical segmentectomy or subsegmentectomy, which can spare more pulmonary parenchyma, are currently being reconsidered by surgeons as an alternative for managing ground-glass opacities, or in situ or minimally invasive adenocarcinoma (2,18,19). Especially for intersegmental nodules, subsegmentectomy can be an ideal surgical approach (20). To some degree, it is undoubtedly that these 3D technologies will be unlikely to meet the future thoracic surgery’s requirements of precision because there is the possibility of missing vessels. Also, virtual reality (VR) technology for the purposes of simulation and training could also be applied in thoracic surgery (21-23).
Twelve years ago, Murota et al. reported that the sub-subsegmental (fifth-order) PA could be visualized using MDCT with MPR images (24). Five years ago, Ghaye et al. analyzed the influence of MDCT on the identification of small arteries and concluded that 94% (1,125 of 1,200) of the subsegmental PA and 74% (1,782 of 2,400) of the sub-subsegmental PA were identified by CT with reconstructed scans of 1.25-mm-thick sections (25). Today, MDCT is already widespread. In the present study, the thin-section MDCT images revealed detailed information of all the arteries (138 of 138), including the small branches. In our opinion, compared with the post-processing of 3D reconstruction software, thin-section MDCT images can directly offer the original and unprocessed information of pulmonary structures for surgeons. Due to the inexperience of the surgeon or the low-quality of CT scans, or other technical reasons, the 3D reconstruction may miss some information, which could lead to an erroneous conclusion. The medico-legal testimony of rib fractures is a good example, as 3D reconstruction is used only in a complementary capacity (26). Hence, surgeons should be familiar with preoperative information concerning anatomical structures using initial CT images and regard the 3D technology as a supplementary means to grasp the dimensions of pulmonary structures.
In conclusion, this study found that both 3D image reconstruction and thin-section MDCT provided precise preoperative information about SA. The 3D image reconstruction software “Exoview” could visualize SAs for surgeons. However, the thin-section MDCT provided a better evaluation of small SA branches. This study also has some limitations. As a single-center sample study, the amount of include cases is low, thus a larger sample is required for further study.
Acknowledgments
Funding: This work was supported by the National Natural Science Foundation of China (81672934 to JZ, 81873417 to CL, 81602704 to CX).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/jtd-20-1014
Data Sharing Statement: Available at http://dx.doi.org/10.21037/jtd-20-1014
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd-20-1014). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This retrospective study was approved by The First Affiliated Hospital of Soochow University (ID: 2020129). All patients enrolled completed the informed consent form.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Hirsch FR, Scagliotti GV, Mulshine JL, et al. Lung cancer: current therapies and new targeted treatments. Lancet 2017;389:299-311. [Crossref] [PubMed]
- Vansteenkiste J, Crino L, Dooms C, et al. 2nd ESMO Consensus Conference on Lung Cancer: early-stage non-small-cell lung cancer consensus on diagnosis, treatment and follow-up. Ann Oncol 2014;25:1462-74. [Crossref] [PubMed]
- Ghaly G, Kamel M, Nasar A, et al. Video-Assisted Thoracoscopic Surgery Is a Safe and Effective Alternative to Thoracotomy for Anatomical Segmentectomy in Patients With Clinical Stage I Non-Small Cell Lung Cancer. Ann Thorac Surg 2016;101:465-72; discussion 472. [Crossref] [PubMed]
- Song CY, Sakai T, Kimura D, et al. Comparison of perioperative and oncological outcomes between video-assisted segmentectomy and lobectomy for patients with clinical stage IA non-small cell lung cancer: a propensity score matching study. J Thorac Dis 2018;10:4891-901. [Crossref] [PubMed]
- Martin-Ucar AE, Nakas A, Pilling JE, et al. A case-matched study of anatomical segmentectomy versus lobectomy for stage I lung cancer in high-risk patients. Eur J Cardiothorac Surg 2005;27:675-9. [Crossref] [PubMed]
- Akiba T. Utility of three-dimensional computed tomography in general thoracic surgery. Gen Thorac Cardiovasc Surg 2013;61:676-84. [Crossref] [PubMed]
- Yao F, Wang J, Yao J, et al. Three-dimensional image reconstruction with free open-source OsiriX software in video-assisted thoracoscopic lobectomy and segmentectomy. Int J Surg 2017;39:16-22. [Crossref] [PubMed]
- Sakurai H, Asamura H. Sublobar resection for early-stage lung cancer. Transl Lung Cancer Res 2014;3:164-72. [PubMed]
- Bendixen M, Jorgensen OD, Kronborg C, et al. Postoperative pain and quality of life after lobectomy via video-assisted thoracoscopic surgery or anterolateral thoracotomy for early stage lung cancer: a randomised controlled trial. Lancet Oncol 2016;17:836-44. [Crossref] [PubMed]
- Ymashita H. Roentgenologic anatomy of the lung. Tokyo: Igaku-Shoin, 1978:70-94.
- Boffa DJ, Dhamija A, Kosinski AS, et al. Fewer complications result from a video-assisted approach to anatomic resection of clinical stage I lung cancer. J Thorac Cardiovasc Surg 2014;148:637-43. [Crossref] [PubMed]
- Paul S, Altorki NK, Sheng S, et al. Thoracoscopic lobectomy is associated with lower morbidity than open lobectomy: a propensity-matched analysis from the STS database. J Thorac Cardiovasc Surg 2010;139:366-78. [Crossref] [PubMed]
- Nakazawa S, Shimizu K, Mogi A, et al. VATS segmentectomy: past, present, and future. Gen Thorac Cardiovasc Surg 2018;66:81-90. [Crossref] [PubMed]
- Watanabe S, Arai K, Watanabe T, et al. Use of three-dimensional computed tomographic angiography of pulmonary vessels for lung resections. Ann Thorac Surg 2003;75:388-92; discussion 392. [Crossref] [PubMed]
- Fukuhara K, Akashi A, Nakane S, et al. Preoperative assessment of the pulmonary artery by three-dimensional computed tomography before video-assisted thoracic surgery lobectomy. Eur J Cardiothorac Surg 2008;34:875-7. [Crossref] [PubMed]
- Akiba T, Marushima H, Harada J, et al. Importance of preoperative imaging with 64-row three-dimensional multidetector computed tomography for safer video-assisted thoracic surgery in lung cancer. Surg Today 2009;39:844-7. [Crossref] [PubMed]
- Murota M, Yamamoto Y, Satoh K, et al. Preoperative Evaluation of the Right Upper Lobe Pulmonary Artery by 3D-CT Pulmonary Angiography vs. Thin-Section Multiplanar Reconstruction Images Obtained by Contrast-Enhanced Multidetector-Row CT. Acta Med Okayama 2015;69:327-32. [PubMed]
- Leighl NB, Curigliano G. Early-stage lung cancer--what do the experts recommend? Ann Oncol 2014;25:1451-3. [Crossref] [PubMed]
- Rami-Porta R, Tsuboi M. Sublobar resection for lung cancer. Eur Respir J 2009;33:426-35. [Crossref] [PubMed]
- Wu WB, Xia Y, Pan XL, et al. Three-dimensional navigation-guided thoracoscopic combined subsegmentectomy for intersegmental pulmonary nodules. Thorac Cancer 2019;10:41-6. [Crossref] [PubMed]
- Marshall MB. Simulation for technical skills. J Thorac Cardiovasc Surg 2012;144:S43-7. [Crossref] [PubMed]
- Solomon B, Bizekis C, Dellis SL, et al. Simulating video-assisted thoracoscopic lobectomy: a virtual reality cognitive task simulation. J Thorac Cardiovasc Surg 2011;141:249-55. [Crossref] [PubMed]
- Trehan K, Kemp CD, Yang SC. Simulation in cardiothoracic surgical training: where do we stand? J Thorac Cardiovasc Surg 2014;147:18-24.e2. [Crossref] [PubMed]
- Murota M, Satoh K, Yamamoto Y, et al. Evaluation of subsubsegmental pulmonary arteries of the posterior and anterior segments of the right upper lobe using multidetector row computed tomography with multiplanar reconstruction images. Jpn J Radiol 2009;27:86-90. [Crossref] [PubMed]
- Ghaye B, Szapiro D, Mastora I, et al. Peripheral pulmonary arteries: how far in the lung does multi-detector row spiral CT allow analysis? Radiology 2001;219:629-36. [Crossref] [PubMed]
- Borowska-Solonynko A, Solonynko B. The use of 3D computed tomography reconstruction in medico-legal testimony regarding injuries in living victims - Risks and benefits. J Forensic Leg Med 2015;30:9-13. [Crossref] [PubMed]


