Association between reproductive lifespan and lung function among postmenopausal women
Introduction
With the increase in life expectancy, women spend a greater proportion of their lifetime in the postmenopausal state (1,2). Menopause is a physiological phase characterized by a decrease in hormone production due to the depletion of ovarian function and cessation of menstruation (3). With menopause, women experience various health problems such as hot flashes, night sweats, vaginal dryness, headache, insomnia, and osteoporosis. Postmenopausal women are often considered as a risk population for chronic diseases such as cardiovascular disease and metabolic diseases including diabetes mellitus and obesity (4-6). Likewise, menopause was associated with impaired lung function along with respiratory symptoms (7-12). In addition, menopause is associated with accelerated lung function decline, especially for forced vital capacity (FVC) beyond the expected age change. The population-based longitudinal European Community Respiratory Health Survey (ECRHS) showed that FVC declined more rapidly among transitional (−10.2 mL/year) and post-menopausal women (−12.5 mL/year) compared with women menstruating regularly (8).
Low levels of estradiol are associated with increased systemic inflammation and lung inflammation, which accelerates lung function decline (13). Studies found that early onset menopause was associated with abnormal lung function (7,14). In a recent study conducted with data from UK Biobank, lower lung function, particularly lower FVC having higher risk of spirometric restriction was associated with cessation of menstruation, especially if it occurs early in life (7). Meanwhile, studies found that early age of menarche was also associated with abnormal lung function (15-17). Among participants in the ECRHS II, women with early menarche (age 10 years or less) had lower FEV1 (adjusted difference, −113 mL) and FVC (adjusted difference, −126 mL) (15). Earlier initiation of menstruation may lead to premature completion of lung development and lower maximally attained lung function (17).
Considering that both age of menarche and menopause were associated with abnormal lung function, reproductive lifespan (RL), a potential marker for the lifetime exposure of female sex hormones, may be associated with lung function. Yet, there is limited data about the association between RL and lung function. This study aims to evaluate the association between RL and lung function among postmenopausal women using the Korea National Health and Nutrition Examination Survey (KNHANES). Main hypothesis is that a shorter RL is associated with abnormal lung function.
Methods
Participants
KNHANES from 2010 to 2015 was used in this study. KNHANES is a cross-sectional survey of a nationally representative noninstitutionalized sample using a stratified, multistage, clustered probability sampling design (18).
The 2010–2015 KNHANES study protocols were approved by the Institutional Review Boards of the Korea Centers for Disease Control and Prevention. Written informed consent was obtained from all participants.
Measurement
KNHANES is composed of three component surveys: health examination, health interview, and nutrition survey. For this study, data from the health examination and health interview was used. Health interview was conducted using a standardized questionnaire by a trained interviewer and detailed physical examination was performed at a mobile examination center (18). All methods were carried out in accordance with the approved guidelines and regulations.
Spirometry was part of the health examination and it was performed according to the recommendations of the American Thoracic Society and European Respiratory Society (19). Absolute values of forced expiratory volume in 1 second (FEV1) and FVC were obtained, and the percentage of predicted values (% predicted) for FEV1 and FVC were calculated using the reference equation obtained on analysis of the representative Korean sample (20). Spirometry-defined lung function was classified as normal (FEV1/FVC ≥0.7 and FVC ≥80% predicted), restrictive ventilatory disorder (FEV1/FVC ≥0.7 and FVC <80% predicted), and obstructive ventilatory disorder (FEV1/FVC <0.7) (21). Restrictive or obstructive ventilatory disorder was considered as having abnormal lung function. Because there could be age related over-diagnosis of obstructive lung disease with fixed ratio, we also conducted a sensitivity analysis using the lower limits of the normal range (LLN) criterion to define abnormal lung function. LLN criterion -defined lung function was classified as normal (FEV1/FVC ≥ LLN and FVC ≥ LLN), restrictive ventilatory disorder (FEV1/FVC ≥ LLN and FVC < LLN), and obstructive ventilatory disorder (FEV1/FVC < LLN) using the reference equations from the KNHANES II (22). Height, weight, and waist circumference were measured by trained nurses. Body mass index (BMI) was calculated by dividing the weight (kg) by the height squared (m) and classified as underweight (<18 kg/m2), normal (18–22.9 kg/m2), overweight (23–24.9 kg/m2), and obese (≥25 kg/m2), according to the World Health Organization guidelines for Asian populations (23). Number of parity was classified into two groups (<4 and ≥4). Comorbid condition included arterial hypertension, dyslipidemia, diabetes mellitus, which were based on laboratory data and self-reports of physician diagnosis from questionnaire conducted by the KNHANES (24,25). All clinical analyses were performed by the Neodin Medical Institute, a laboratory certified by the Korea Ministry of Health and Welfare.
For RL and endogenous hormone-related factors including number of parity, or use of oral contraceptive, the data was obtained from the health interview. RL (years) was calculated by subtracting the age at menarche from age at menopause. Since there was no cut-off for RL, we categorized it into 3 groups according to the sample distribution: 10th percentile (≤30 years), 11th–89th percentiles (>30 to <39 years), and 90th percentile (≥39 years). Age at menarche and age at menopause were categorized into 3 groups: 10th percentile (≤13 years), 11th–89th percentile (>13 to <18 years), and 90th percentile (≥18 years); 10th percentile (≤46 years), 11th–89th percentile (>46 to <54 years), and 90th percentile (≥54 years), respectively. Smoking history was divided into 3 categories: current or former smoker (those who had smoked 100 or more cigarettes over their lifetimes and did or did not smoke currently respectively) and never smoker (those who had smoked 100 or less cigarettes over their lifetimes). In addition, information on scholarly level, monthly family income, marital status, smoking status, medical history, and comorbidities were obtained from the health interview. Comorbid conditions also included tuberculosis, asthma, stroke, myocardial infarction, cancer, osteoarthritis, and rheumatoid arthritis.
Statistical methods
The KNHANES sampling weights accounting for the complex survey design, survey non-response and post-stratification were used (18). Descriptive statistics were used to summarize the characteristics of participants by the presence of abnormal lung function. Continuous and categorical variables were compared among the two categories of group using linear regression and χ2 tests, respectively. Nominal variables were presented as a number and percentage, and continuous variables were presented as a mean ± standard error of the mean.
Multinomial logistic regression to evaluate the association between RL and abnormal lung function was used, and participants with normal lung function were used as the reference group. For the main analyses, the multivariable-adjusted prevalence ratios (PR) and 95% confidence intervals (CI) of abnormal lung function prevalence comparing RLs were calculated. The same analyses were performed for age at menarche and menopause. Age, BMI categories, smoking status, cardiovascular disease, diabetes mellitus, musculoskeletal disease, asthma, tuberculosis, oral contraceptive use, and number of high parity were adjusted to control potential confounders.
In addition to categorical analysis, RL was modeled as continuous variables using restricted cubic splines with knots at the 5th, 35th, 65th, and 95th percentiles of the sample distribution to provide a flexible estimate of the dose-response relationship between RL and predictive FVC and FEV1. Stratified analyses were also performed to evaluate the association of RL and abnormal lung function in pre-specified subgroups defined by obesity (no and yes) and smoking status (never and ever).
P value <0.05 was considered as statistically significant. All analyses were performed using the STATA version 14 (StataCorp LP, College Station, TX, USA) and R 3.2.1 (Vienna, Austria; http://www.R-project.org/).
Results
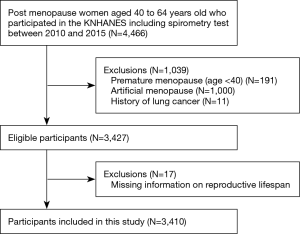
Postmenopausal women aged 40 to 64 years old who underwent spirometry (N=4,466) were included. Women who experienced premature menopause (<40 years; N=191), artificial menopause (N=1,000), or history of lung cancer (N=11) were excluded. In addition, 17 participants who had missing information regarding age at menarche or menopause onset were excluded. The final sample size was 3,410 (Figure 1).
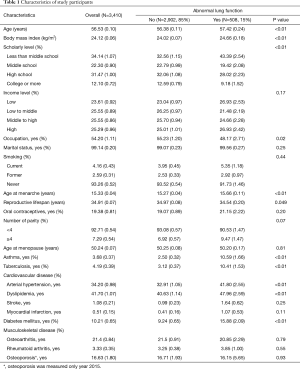
Among 3,410 postmenopausal women, the mean (standard error) age at survey was 56.53 (0.10). The mean menarche age, menopause age, and RL was 15.33 (0.04), 50.24 (0.07), 34.91 (0.07), respectively (Table 1).
Full table
The prevalence of abnormal lung function was 15.0% (Table 1). Women with abnormal lung function were older (57.42 vs. 56.38 years, P<0.01), and more likely to have higher BMI (24.66 vs. 24.02, P<0.01), asthma (10.59% vs. 2.5%, P<0.01), tuberculosis (10.41% vs. 3.12%, P<0.01), arterial hypertension (41.80% vs. 32.91%, P<0.01), myocardial infarction (1.07% vs. 0.41%, P=0.11), and diabetes mellitus (15.88% vs. 9.24%, P<0.01) than those with normal function. Women with abnormal lung functions reported later menarche (15.66 vs. 15.27 years, P<0.01) and shorter RL (34.54 vs. 34.97 years, P=0.049) than women with normal lung function.
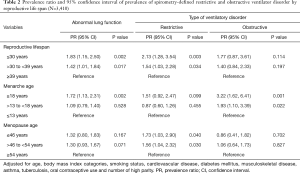
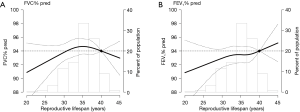
In adjusted multivariable models, the PRs for abnormal lung function in women with RL ≤30 years and >30 to <39 years compared to that in women with ≥39 years of RL were 1.83 and 1.42, respectively (Table 2). In spline regression models, the association between RL (years) and the predicted FVC and FEV1 was linear, with reduced function across shorter RL (Figure 2). Women who experienced menarche ≥18 years had 1.72 times higher prevalence of abnormal lung function than woman who experienced menarche ≤13 years. Women with menopausal age ≤46 years had 1.32 times higher prevalence of abnormal lung function than women with menopausal age ≥54 years (Table 2).
Full table
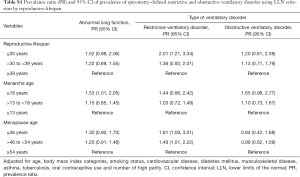
In the fully adjusted model, women with ≤30 years of RL showed a 2.13- and 1.54-time higher prevalence of restrictive and obstructive ventilatory disorder, respectively, than women with ≥39 years of RL. Women who experienced menarche ≥18 years were more likely to have an obstructive ventilatory disorder than women who experienced menarche ≤13 years. Women with menopausal age ≤46 years were more likely to have a restrictive ventilatory disorder than women with menopausal age ≥54 years (Table 2). In the sensitivity analysis using LLN criterion, the results were similar (Table S1).
Full table
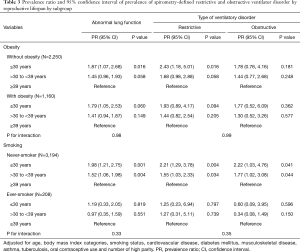
The positive association between shorter RL and higher prevalence of abnormal lung function was stronger in women without obesity and non-smoking status, but the interactions were not statistically significant (all P values for interactions >0.05; Table 3).
Full table
Discussion
With nationally representative sampled data, shorter RL was associated with abnormal lung function, in particular, restrictive ventilatory disorder. The relationship seemed more robust among non-obese and non-smoking women, but the interactions were not statistically significant. Late onset of menarche was associated with abnormal lung function, particularly with an obstructive ventilatory disorder, and early menopause was associated with restrictive ventilatory disorder suggesting that there would be several mechanisms through which female hormones affect lung function.
In this study, shorter RL was associated with restrictive ventilatory disorder. While there have been many studies evaluating the association either between age of menopause or menarche and lung function, there was no study regarding the association between RL and lung function. Women who had a RL ≤30 years had about 1.8 times higher risk of having restrictive ventilatory disorder compared to women who had ≥39 years of RL. Women with ≤30 years of RL would have reduced exposure to estradiol compared to women with ≥39 years of RL. The restrictive ventilatory disorder may be due to similar mechanisms of abnormal lung function in postmenopausal women. Estradiol has been demonstrated to have an anti-inflammatory effect (13). In line, the hypoestrogenism associated with changes in the hypothalamo-pituitary-gonadal axis in menopause is associated with increased systemic inflammation and lung inflammation, which may contribute to deteriorating lung function (13,26). Further, the decrease in estradiol increases the risk of osteoporosis, which causes compression of the vertebra. This interferes with the lung expansion, resulting in a decrease in lung function, particularly FVC (27). Moreover, postmenopausal women become more insulin resistant (5,28), which is associated with lower lung function and increased respiratory symptoms (29-31). Women with shorter RLs may be more likely to be insulin resistant, resulting in lower lung function. However, the mechanisms underlying associations between RL and lung function impairment need to be further investigated.
While the interactions were not statistically significant, we found that non-obese and non-smoking women with shorter RLs were at a bit higher risk of restrictive ventilator disorder. Hormonal status might not be a very strong predictor of abnormal lung function among postmenopausal women when other important comorbidities such as obesity and smoking are also present. In fact, previous studies showed that a restrictive ventilator disorder, represented by a low FVC, was not only associated with cardiovascular diseases (32), but also with a multitude of clinical conditions, such as aging (33), smoking (34), obesity, metabolic syndrome, and diabetes mellitus (35,36). In line with low FVC under systemic influence, the relationship of RL with cardiovascular diseases has been previously suggested. Risk of coronary heart disease and cardiovascular mortality were lower among women with a higher age at menopause (37-39) and one study observed a reduction in cardiovascular mortality among women with longer exposure to endogenous estrogen (39). Another study showed that shorter RL was associated with an increased risk of noncardioembolic stroke (40). Further studies are necessary to elucidate the relationships among RL, lung function, and cardiovascular disease risk. Yet, considering the burden of pulmonary and cardiovascular diseases in middle- and older-aged women, health professionals need to consider screening and monitoring lung and cardiac function in postmenopausal women irrespective of obesity and smoking status.
In this study, late onset of menarche was associated with abnormal lung function, particularly an obstructive ventilatory disorder. The subjects with late age of menarche (≥18 years) were significantly older than other groups. Considering that half of them were born during the Korean war, there may be birth cohort effects, such as malnutrition during the war, on abnormal lung growth and development (41). This submaximal lung growth and development could be one possible explanation for the relationship between late onset of menarche and obstructive ventilatory disorder (42). Nevertheless, results in this study are different from those of previous studies. Early onset of menarche has been considered one of the clinical factors associated with increased risk of asthma or abnormal lung function, particularly low FVC (15-17,43-45). The multinational ECRHS also showed that lower lung function measured by FEV1 and FVC was more common in women with early menarche, and abnormal lung health mainly appeared to be related to menarche before the age of 11 years (15). However, in this study, there were only a few subjects who had menarche before the age of 11 years, and it was not possible to evaluate the association.
We also found that restrictive ventilatory disorder was associated with the early onset of menopause. This is consistent with the results from a previous large multicenter population-based study which used the UK Biobank data. The study reported that women with early menopause had lower FVC and higher risk of spirometric restriction among postmenopausal women, but early onset of menopause was not associated with airflow obstruction (7). Given that change of FVC was more pronounced than the change of FEV1 among transitional and postmenopausal women in the previous study (8), restrictive ventilatory disorder determined by FVC may be more associated with early onset of menopause than obstructive ventilatory disorder determined by FEV1.
There were several limitations in this study. First, this study was a cross-section design, which limits the assessment of causal inference between abnormal lung function and reproductive life span. As reverse causality for the impact of abnormal lung function on early onset of menarche or menopause could be possible, further studies with longitudinal follow-up analyses need to be conducted. Secondly, data on age of menarche and menopause onset were based on questionnaires, so there was a possibility of measurement errors. However, as age of menarche and menopause onset mark important points to women, this measurement error would be small. Thirdly, the RL acquired from questionnaires may also have measurement errors despite adjustment for the number of high parity and the use of oral contraceptive or hormone replacement therapy. Fourth, measurement of total lung capacity (TLC) to determine accurate restrictive ventilatory disorder was not available in the KNHANES data and some women with obstructive ventilatory disorder might be classified as having restrictive ventilatory disorder. Further studies with comprehensive lung function tests including TLC are needed to validate our study findings. In addition, information for hazardous substances at the workplace, which might affect lung function, was lacking. However, there were more participants with abnormal lung function among women who did not have a job at the time of survey. Finally, this study used a nationwide representative sample of Koreans, which might not be generalizable to subjects in different countries or of other ethnicities. Despite these limitations, the national representative nature of the patient sample considered in conjunction with the strict quality control procedures of the Korea NHANES is a major strength of this study, adding plausibility to findings.
Conclusions
In conclusion, we demonstrated that shorter RL in postmenopausal women is associated with a decrease in lung function, particularly with a restrictive ventilatory disorder. Clinicians should pay attention to respiratory impairment as well in postmenopausal women, especially with short female hormonal exposure.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd-19-3726). HYP serves as an unpaid editorial board member of The Journal of Thoracic Disease from Feb, 2019 to Jan, 2021. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The 2007–2015 KNHANES study protocols were approved by the Institutional Review Boards of the Korea Centers for Disease Control and Prevention (No. 2007-02CON-04-P, 2008-04EXP-01-C, 2009-01CON-03-2C, 2010-02CON-21-C, 2011-02CON-06-C, 2012-01EXP-01-2C, 2013-07CON-03-4C, 2013-12EXP-03-5C, and 2015-01-02-6C). All patients enrolled completed the informed consent form.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Organization WH. Report of a WHO Scientific Group. Research on the Menopause in the 1990s. WHO technical report series 1996:866. Available online: https://apps.who.int/iris/handle/10665/41841
- Organization WH. World health statistics 2016: monitoring health for the SDGs sustainable development goals. World Health Organization; 2016. Available online: https://www.who.int/gho/publications/world_health_statistics/2016/en/
- Speroff L, Glass RH, Kase NG. Menopause and the perimenopausal transition. In: Mitchell C. editor. Clinical gynecologic endocrinology and infertility. 6th edition. Baltimore: Lippincott Williams & Wilkins, 1999:643-724.
- Bauld R, Brown RF. Stress, psychological distress, psychosocial factors, menopause symptoms and physical health in women. Maturitas 2009;62:160-5. [Crossref] [PubMed]
- Carr MC. The emergence of the metabolic syndrome with menopause. J Clin Endocrinol Metab 2003;88:2404-11. [Crossref] [PubMed]
- Kuh D, Langenberg C, Hardy R, et al. Cardiovascular risk at age 53 years in relation to the menopause transition and use of hormone replacement therapy: a prospective British birth cohort study. BJOG 2005;112:476-85. [Crossref] [PubMed]
- Amaral AF, Strachan DP, Gómez Real F, et al. Lower lung function associates with cessation of menstruation: UK Biobank data. Eur Respir J 2016;48:1288-97. [Crossref] [PubMed]
- Triebner K, Matulonga B, Johannessen A, et al. Menopause is associated with accelerated lung function decline. Am J Respir Crit Care Med 2017;195:1058-65. [Crossref] [PubMed]
- Songür N, Aydin ZD, Oztürk O, et al. Respiratory symptoms, pulmonary function, and reproductive history: Isparta Menopause and Health Study. J Womens Health (Larchmt) 2010;19:1145-54. [Crossref] [PubMed]
- Real FG, Svanes C, Omenaas ER, et al. Lung function, respiratory symptoms, and the menopausal transition. J Allergy Clin Immunol 2008;121:72-80.e3. [Crossref] [PubMed]
- Hayatbakhsh MR, Najman JM, O’Callaghan MJ, et al. Association between smoking and respiratory function before and after menopause. Lung 2011;189:65-71. [Crossref] [PubMed]
- Jarvis D, Leynaert B. The association of asthma, atopy and lung function with hormone replacement therapy and surgical cessation of menstruation in a population‐based sample of English women. Allergy 2008;63:95-102. [PubMed]
- Straub RH. The complex role of estrogens in inflammation. Endocr Rev 2007;28:521-74. [Crossref] [PubMed]
- Jacobsen BK, Heuch I, Kvåle G. Age at natural menopause and all-cause mortality: a 37-year follow-up of 19,731 Norwegian women. Am J Epidemiol 2003;157:923-9. [Crossref] [PubMed]
- Macsali F, Real FG, Plana E, et al. Early age at menarche, lung function, and adult asthma. Am J Respir Crit Care Med 2011;183:8-14. [Crossref] [PubMed]
- Macsali F, Svanes C, Bjørge L, et al. Respiratory health in women: from menarche to menopause. Expert Rev Respir Med 2012;6:187-202. [Crossref] [PubMed]
- Gill D, Sheehan NA, Wielscher M, et al. Age at menarche and lung function: a Mendelian randomization study. Eur J Epidemiol 2017;32:701-10. [Crossref] [PubMed]
- Kweon S, Kim Y, Jang MJ, et al. Data resource profile: the Korea National Health and Nutrition Examination Survey (KNHANES). Int J Epidemiol 2014;43:69-77. [Crossref] [PubMed]
- Miller M. ATS/ERS task force: standardisation of spirometry. Eur Respir J 2005;26:319-38. [Crossref] [PubMed]
- Choi J, K., Paek D, Lee JO. Normal predictive values of spirometry in Korean population. Tuberc Respir Dis 2005;58:230-42. [Crossref]
- Sim YS, Lee JH, Lee WY, et al. Spirometry and Bronchodilator Test. Tuberc Respir Dis (Seoul) 2017;80:105-12. [Crossref] [PubMed]
- Hwang YI, Kim CH, Kang HR, et al. Comparison of the prevalence of chronic obstructive pulmonary disease diagnosed by lower limit of normal and fixed ratio criteria. J Korean Med Sci 2009;24:621-6. [Crossref] [PubMed]
- Organization WH. The Asia-Pacific perspective: redefining obesity and its treatment. Sydney: Health Communications Australia; 2000. Available online: https://apps.who.int/iris/handle/10665/206936
- Bibbins-Domingo K, Grossman DC, Curry SJ, et al. Statin use for the primary prevention of cardiovascular disease in adults: US Preventive Services Task Force recommendation statement. JAMA 2016;316:1997-2007. [Crossref] [PubMed]
- Lee H, Shin SH, Gu S, et al. Racial differences in comorbidity profile among patients with chronic obstructive pulmonary disease. BMC Med 2018;16:178. [Crossref] [PubMed]
- Chotirmall SH, Greene CM, Oglesby IK, et al. 17β-estradiol inhibits IL-8 in cystic fibrosis by up-regulating secretory leucoprotease inhibitor. Am J Respir Crit Care Med 2010;182:62-72. [Crossref] [PubMed]
- Leech JA, Dulberg C, Kellie S, et al. Relationship of lung function to severity of osteoporosis in women 1-3. Am Rev Respir Dis 1990;141:68-71. [Crossref] [PubMed]
- Kalish GM, Barrett-Connor E, Laughlin GA, et al. Association of endogenous sex hormones and insulin resistance among postmenopausal women: results from the Postmenopausal Estrogen/Progestin Intervention Trial. J Clin Endocrinol Metab 2003;88:1646-52. [Crossref] [PubMed]
- Lange P, Groth S, Kastrup J, et al. Diabetes mellitus, plasma glucose and lung function in a cross-sectional population study. Eur Respir J 1989;2:14-9. [PubMed]
- Engström G, Hedblad B, Nilsson P, et al. Lung function, insulin resistance and incidence of cardiovascular disease: a longitudinal cohort study. J Intern Med 2003;253:574-81. [Crossref] [PubMed]
- Lawlor DA, Ebrahim S, Smith GD. Associations of measures of lung function with insulin resistance and type 2 diabetes: findings from the British Women’s Heart and Health Study. Diabetologia 2004;47:195-203. [Crossref] [PubMed]
- Kang HK, Park HY, Jeong BH, et al. Relationship Between Forced Vital Capacity and Framingham Cardiovascular Risk Score Beyond the Presence of Metabolic Syndrome: The Fourth Korea National Health and Nutrition Examination Survey. Medicine (Baltimore) 2015;94:e2089. [Crossref] [PubMed]
- Vaz Fragoso CA, McAvay G, Van Ness PH, et al. Phenotype of Spirometric Impairment in an Aging Population. Am J Respir Crit Care Med 2016;193:727-35. [Crossref] [PubMed]
- Lederer DJ, Enright PL, Kawut SM, et al. Cigarette smoking is associated with subclinical parenchymal lung disease: the Multi-Ethnic Study of Atherosclerosis (MESA)-lung study. Am J Respir Crit Care Med 2009;180:407-14. [Crossref] [PubMed]
- Leone N, Courbon D, Thomas F, et al. Lung function impairment and metabolic syndrome: the critical role of abdominal obesity. Am J Respir Crit Care Med 2009;179:509-16. [Crossref] [PubMed]
- Lee HM, Chung SJ, Lopez VA, et al. Association of FVC and total mortality in US adults with metabolic syndrome and diabetes. Chest 2009;136:171-6. [Crossref] [PubMed]
- Jacobsen BK, Knutsen SF, Fraser GE. Age at natural menopause and total mortality and mortality from ischemic heart disease: the Adventist Health Study. J Clin Epidemiol 1999;52:303-7. [Crossref] [PubMed]
- Jacobsen BK, Nilssen S, Heuch I, et al. Does age at natural menopause affect mortality from ischemic heart disease? J Clin Epidemiol 1997;50:475-9. [Crossref] [PubMed]
- de Kleijn MJ, van der Schouw YT, Verbeek AL, et al. Endogenous estrogen exposure and cardiovascular mortality risk in postmenopausal women. Am J Epidemiol 2002;155:339-45. [Crossref] [PubMed]
- de Lecinana MA, Egido J, Fernandez C, et al. Risk of ischemic stroke and lifetime estrogen exposure. Neurology 2007;68:33-8. [Crossref] [PubMed]
- Speroff L, Fritz MA. Clinical gynecologic endocrinology and infertility. Lippincott Williams & Wilkins, 2005.
- Lange P, Celli B, Agusti A, et al. Lung-Function Trajectories Leading to Chronic Obstructive Pulmonary Disease. N Engl J Med 2015;373:111-22. [Crossref] [PubMed]
- Varraso R, Siroux V, Maccario J, et al. Asthma severity is associated with body mass index and early menarche in women. Am J Respir Crit Care Med 2005;171:334-9. [Crossref] [PubMed]
- Salam MT, Wenten M, Gilliland FD. Endogenous and exogenous sex steroid hormones and asthma and wheeze in young women. J Allergy Clin Immunol 2006;117:1001-7. [Crossref] [PubMed]
- Al-Sahab B, Hamadeh MJ, Ardern CI, et al. Early menarche predicts incidence of asthma in early adulthood. Am J Epidemiol 2011;173:64-70. [Crossref] [PubMed]