
Efficient isolation and quantification of circulating tumor cells in non-small cell lung cancer patients using peptide-functionalized magnetic nanoparticles
Introduction
Lung cancer is the most common malignant tumor with the highest morbidity and mortality (1). Approximately 80% of lung cancer patients are diagnosed with non-small cell lung cancer (NSCLC), with 92% 5-year overall survival in IA1 and only 0% in IVB. Only early detection and diagnosis of lung cancer could reduce mortality and improve quality of life (2). Several tumor markers have been used in clinic but with low sensibility and high false negatives such as carcinoembryonic antigen (CEA) (3). The National Lung Screening Trial (NLST) reported that in the low-dose CT group, the positive predictive value for positive screening results overall was only 3.8% while the value increased from 0.5% to 41.3% as the diameter of the nodule increased from 4 to 6 mm to more than 30 mm. Overall, with low-dose CT, the negative predictive value was 99.9% (4). Due to the high rate of false positives or false negatives, and considering the risk of radioactive exposure, the traditional tumor detection methods are not ideal for the detection of lung cancer in the early stage. Therefore, we need to discover new tumor markers for the early detection and diagnosis of lung cancer.
Cells from early, low-density lesions displayed more stemness features, migrated more and founded more metastases than cells from dense, advanced tumors have been found in breast cancer and lung cancer (5). In recent years, more and more attention has been paid to the role of “liquid biopsy” in early detection, disease progression, recurrence and metastasis, and prognosis monitoring of tumors. Assessing circulating tumor cells (CTCs), which CTCs were first discovered in peripheral blood of a patient with metastatic cancer in 1869 (6), counts using liquid biopsies as a whole, or as specific subgroups, could enhance understanding of disease progression and has been shown to carry prognostic information in several cancer types, including lung cancer CTCs are cells that have shed into the blood circulation system from a primary tumor (7-13). It spreads to the different body parts through blood circulation, proliferates in the appropriate environment, and leads to recurrence and metastasis of tumors (14). Many researches reveal that CTCs play an important role in the occurrence, progression and metastasis of breast cancer, colorectal cancer, prostate cancer and other cancers.
CTCs can be isolated and quantified at all stages in lung cancer (15). In early stage lung cancer, CTCs analysis might provide prognostic information in resectable patients (16,17). In advanced NSCLC, CTCs have been proposed to be an independent prognostic marker and the higher CTC numbers is associated with worse prognosis (18,19). It has been reported that after chemotherapy for advanced lung cancer, CTC numbers can reflect subtle metastases and recurrent tumors which are difficult to detect by imaging (20). However, due to the limited detection of CTCs in NSCLC at present, there are not many studies and applications of CTCs, which can facilitate treatment and disease monitoring in NSCLC (21).
The extensive applications of CTC detection play a vital role in tumor study. The gold standard of CTC detection is the CellSearch® (Veridex, Raritan, NJ, USA) System, which is the current clinical standard for CTC enumeration in breast, prostate and colorectal cancer. This system is based on the principle of specific immunological recognition of epithelial cell adhesion molecule (EpCAM)-positive cells. The sensitivity of CellSearch was quite low in every cancer species, around 10% to 60% (prostate cancer was 54.1%; breast cancer was 49.2%) (22-28). The low sensitivity limits the use of CellSearch system in NSCLC is also reported in late stage NSCLC from 31.7% to 78% (12,29-32). Only few researches revealed that the sensitivity of early stage lung cancer varies from 30% to 40% (17,33). Therefore, for more accurate and informative studies on NSCLC, a novel and more effective detection system for CTCs is crucial.
Previously, our team reported an innovative CTC detection method (TumorFisher) based on the iron oxide magnetic nanoparticles functionalized with EpCAM recognition peptide (Pep). The rate of capture efficiency and purity were up to 90% and 93% in breast cancer, prostate cancer and liver cancer cell lines and patients (34-36), but in lung cancer remain unknown. In this study, we applied this technique to lung cancer cell lines and all stage lung cancer patients and compared with CellSearch.
Method
Patients and blood sample collection
Eighty-eight consecutive patients who underwent surgery or biopsy for NSCLC between August 2016 and November 2017 at the Department of Thoracic Surgery, Peking Union Medical College Hospital (PUMCH) were included in our study. The patients received the necessary information concerning the study, and consent was obtained from each of them. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and was approved by the PUMCH ethics committees (JS-1263). Morphologic classification was assigned according to WHO and AJCC criteria (37). The tumors were staged according to the 8th edition of the international tumor-node-metastasis (TNM) system (2). All the patients were drawn fasting blood samples (2 mL) in the morning within 24 h before surgery or biopsy with treatment naïve.
Cell culture
Two typical NSCLC derived A549 and NCI-H1975 cell lines were purchased from the Cell Resource Center of Chinese Academy of Medical Sciences (Beijing, China). The two cell lines were selected to evaluate the binding affinity and capture efficiency between the two systems prior the clinical experiment. The two cell lines were cultured in a flask containing RPMI-1640 medium with 10% heat-inactivated FBS and 100 U/mL penicillin and 100 µg/mL streptomycin. The cell culture incubator was set to a humidified atmosphere with 5% CO2 at 37 °C. Media was renewed every three days. The cells were harvested till the confluence reached about 80-90%. After washed twice with PBS buffer, 1 mL of 0.25% trypsin-EDTA was added for 3 min under 37 °C for trypsinization in order to detach the cells from the bottom of the flask. Then, 2 mL of RPMI-1640 complete medium was added to neutralized the trypsin. The cells were finally separated by centrifuging at 1,000 rpm for 5 min. Finally, the supernatant was discarded and the suspended cells were enumerated.
Preparation of functionalized Pep@MNPs and anti-EpCAM@MNPs
Specifically, 2 mg iron oxide magnetic nanoparticles modified with streptavidin (MNPs) with diameter of 150±30 nm and concentration of 5 mg/mL were incubated with 0.5 mg biotin-conjugated Pep or 0.1 mg biotin-conjugated anti-EpCAM antibody in 1 mL PBS for 1 h with continuous shaking at room temperature. Then, the functionalized Pep@MNPs and anti-EpCAM@MNPs were washed twice with PBS, and dispersed in 1 mL of PBS, and finally stored in PBS (containing 0.05% NaN3) at 4 °C. Anti-EpCAM@MNPs was used as positive control for Pep@MNPs.
The binding affinity measurement by flow cytometry
Flow cytometry was used for analyzing binding affinity of Pep and anti-EpCAM antibody to typical NSCLC A549 and NCI-H1975 cells. Harvested cells were resuspended in a 2 mL EP tube and fixed with 4% paraformaldehyde for 15 min. After washed with PBS two times, cells were blocked with 4% bovine serum albumin (BSA) for 30 min. Then 15 µL 1 mg/mL FITC-labeled Pep was added to cell suspension with about 105 suspended tumor cells for 1 h with continuous shaking at room temperature. After washed with PBS 3 times, the fluorescence signal was detected using a C6 Accuri flow cytometer (BD Biosciences, USA). FITC-labeled anti-EpCAM monoclonal antibody and mouse IgG2bκ-FITC were used as positive controls and isotype controls, respectively. Fluorescent distributions were measured by C6 flow cytometer for calculation of the binding affinity between Pep and tumor cells (numbers of FITC-labeled tumor cells/numbers of the total tumor cells added).
Evaluation of capture efficiency in cell lines
Added separately 10 µL Pep@MNPs and anti-EpCAM@MNPs into 2 mL EP tubes with 1 mL PBS containing about 1,000 cells. The above system was incubated for 1 h with continuous shaking at 37 °C and washed under a 116 mT magnetic field for 3 times. The captured cell samples were incubated with phycoerythrin (PE)-labeled anti-CD45 and AF488-labeled anti-CK for 1 h at 37 °C and followed by PBS washing at least 3 times. Then 1 µL DAPI at concentration of 100 µg/mL was added for cell nuclear staining. The Olympus IX 73 fluorescence microscopy was employed to count and measure the capture efficiency (numbers of identified tumor cells/numbers of added tumor cells). The above same procedures were applied for both A549 and NCI-H1975 cell lines.
CTC capture and detection
Blood samples were collected in 10 mL CellSave (Veridex) preservative tubes, stored at room temperature, and processed within 96 h of collection, according to the manufacturer’s instructions. CTCs from NSCLC patients’ blood samples were captured and enumerated by TumorFisher and CellSearch technologies, respectively. The TumorFisher technology refer to the prior established Pep@MNPs CTC enumeration system. Briefly, 10 µL Pep@MNPs were added into 2 mL blood samples and incubated for 1 h at 37 °C. The captured cells were gently washed with PBS at least 3 times under a high magnetic field (116 mT). The dying proceeding was the same as for cell lines above. CTC were detected by further quantifying expression levels of CK and CD45 in individual cells. CTCs exhibit strong CK expression and negligible CD45 signals. In contrast, white blood cells (WBCs) present low CK and high CD45 expression levels. DAPI staining validates that the captured cells retain intact nuclei. The combined information was utilized to identify CTCs (DAPI+/CK+/CD45−) from WBCs (DAPI+/CK−/CD45+) and cellular debris. CTCs was counted by identifying the number of captured cells (DAPI+/CK+/CD45−) in 2 mL blood.
The CellSearch isolation procedures were conducted according to the manufacture’s instruction. Approximately 6.5 mL of dilution buffer was added and mixed with 7.5 mL of blood sample. The mixture then was transferred into CellTracks AutoPrep system. The anti-EpCAM antibodies coated magnetic beads were used in the remaining isolation procedures, which conducted automatically by the instrument.
Statistical analysis
Statistical analysis was performed using SPSS software, version 24.0 (SPSS Inc., Armonk, USA). Binding affinity and capture efficiency were compared using a two-tailed Student’s t-test. A two-sided P<0.05 was considered to be statistically significant.
Result
Binding affinity and capture efficiency
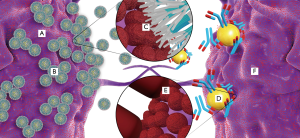
EpCAM is a widely used biomarker for CTC detection in breast, liver, lung carcinoma and other metastatic cancers. In the previous studies, we have reported the recognition Peps specifically binding with EpCAM with relatively high binding affinity (35). The schematic illustration of the TumorFisher (left side) and CellSearch (right side) methods are presented in Figure 1 for demonstrating the different mechanisms for capture of CTCs from blood. In the middle zoom-in images, it is clearly observed that much more Peps (the white and light blue strings) interact with EpCAM molecules (brown rough spheres), while much less anti-EpCAM antibody interacts with EpCAM molecules. The different density of surface modification could be ascribed to the huge difference between molecular weight of Pep (2.7 KDa) and antibody (around 150 KDa). The bundle-like Peps as shown in zoom-in image (C in Figure 1) could be attributed to the self-assembly behavior of the N-terminal Pep segments, Asn-Asn-Cys-Asn-Asn-Gly-Gly-Cys between cysteine disulfide linkage and the hydrogen bonds between sidechains on Asn (37-39). Thus, more Pep-magnetic nanoparticles (TumorFisher, fuzzy spherical particles denoted as B in Figure 1) could be adsorbed onto the CTC surface due to the stronger interaction between EpCAM highly expressed CTCs and Pep-magnetic nanoparticles than that between antibody-magnetic beads and CTC. In contrast, it could be observed that less magnetic beads functionalized with anti-EpCAM (D in Figure 1, yellow sphere with randomly oriented Y-shaped antibodies) are adsorbed on the CTC cell surface possibly because less antibody density on bead surface.
The flow cytometry was used to calculate the binding affinity in cell lines. As shown in Figure 2A, the binding affinities of Pep to A549 and NCI-H1975 are 76.7%±11.0% and 70.1%±4.8% respectively, which is slightly lower than the positive control group (anti-EpCAM to A549, 90.6%±4.9%) or similar (anti-EpCAM to NCI-H1975, 67.2%±3.0%). There is no statistical difference in the binding affinity of Pep and anti-EpCAM to A549 and NCI-H1975 (P>0.05). In the isotype controls group, the binding affinities of mouse IgG2bκ-FITC are 0.9%±0.3% and 1.1%±0.9% respectively. Meanwhile, in the negative group, the binding affinities of blank to A549 and NCI-H1975 is 0.3% and 0.6%, respectively. Typical images of A549 and NCI-H1975 flow fluorescence distribution were shown in Figure 2B and Figure 2C.
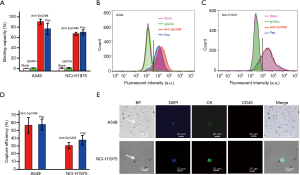
Moreover, A549 and NCI-H1975 cell lines were used to evaluate the capture efficiency of Pep@MNPs and anti-EpCAM@MNPs in isolating CTCs from mimicking CTC samples. As shown in Figure 2D, the capture efficiencies of Pep@MNPs and anti-EpCAM@MNPs from A549 are 57.3%±7.0% and 56.3%±10.1%, respectively. And the capture efficiencies of Pep@MNPs and anti-EpCAM@MNPs from NCI-H1975 are 37.3%±6.1% and 30.3%±4.0%, respectively. The results indicated that the capture efficiency of Pep@MNPs and anti-EpCAM@MNPs to A549 and NCI-H1975 were comparable, and there is no statistical difference.
The conventional definition of CTCs was adopted in this study. Cells that DAPI+/CK+/CD45− and met the phenotypic morphological characteristics were designated as CTCs, and DAPI+/CK−/CD45+ cells were designated as leukocytes. We imaged the cells on an inverted microscope. Figure 2E shows typical immune-fluorescence staining results for isolated A549 (top row) and NCI-H1975 (button row) by Pep@MNPs assay.
Comparation between TumorFisher and CellSearch systems in clinical applications
In order to compare the clinical effect of TumorFisher and CellSearch system, 7 patients with NSCLC were enrolled in this study. The patient characteristics are summarized in Table 1. Among them, 6 patients were in stage I, and 1 patient was in stage II. Patients were drawn blood in morning for blood samples from elbow veins, the blood samples were separated and analyzed with TumorFisher and CellSearch system. According to our research data, the most appropriate cut-off value of the TumorFisher system is 1 cell/2 mL blood, if the patient detected ≥1 CTCs in 2 mL of blood, we would define it positive, while the cut-off value of CellSearch is 5 cells/7.5 mL (40). Thus, the efficiency of TumorFisher is 5/7 (71.4%), which is much higher than CellSearch (0/7, 0%). It is worth mentioning that the false negative of TumorFisher (28.6%) is much lower than CellSearch (100%), as shown in Figure 3A. The representative immunofluorescent images of CTC and WBC found in NSCLC patients were shown as Figure 3B. The three-color immunocytochemistry method, based on DAPI nuclear staining (blue color, Em =461 nm), AF488-labeled anti-CK (green color, Em =525 nm), PE-labeled anti-CD45 (red color, Em =578 nm), was applied to identify CTCs from non-specifically trapped WBC cells. The larger cell size and nuclear size of CTCs than the WBCs are clearly shown in the images, and the DAPI+/CK+/CD45− cells with intact cell features are identified as CTCs and the DAPI+/CK−/CD45+ ones as WBCs.
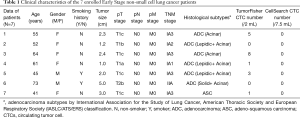
Full table
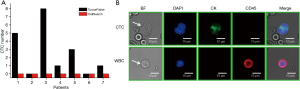
The applications of TumorFisher in clinic
To further explore the clinical value of CTC counting in different stages of disease, 81 patients with stage I–IV NSCLC were enrolled in this study from August 2016 to November 2017 in Department of Thoracic Surgery, PUMCH. The patient characteristics are summarized in Table 2. Of the patients enrolled, 29 were in stage I, 22 were in stage II, 21 were in stage III, and 9 were in stage IV. There were 67 patients with adenocarcinoma, accounting for 82.7% of the total number. After statistical analysis, we found that smoking and non-smoking patients have significant differences in detection rates (P=0.0231), the detection rate of smoker and non-smoking patients are 37% and 63%, respectively. And there is no significant difference between other factors.

Full table
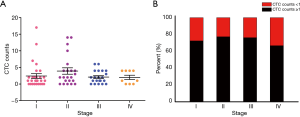
As shown in Figure 4A, the CTC numbers of NSCLC patients varied with different stages. Each point in the figure represents the CTC numbers detected by one patient, and different colors are used to distinguish patients at different disease stages. In this clinical cohort, the overall detection rate of TumorFisher was 59/81 (72.8%), the detection rates of adenocarcinoma and squamous carcinoma were 52/67 (77.6%) and (9/14, 64.3%), respectively. There was almost no difference in the detection rate among patients in stages I, II, and III, and they are maintained at high level (Figure 4B). The detection rates of TumorFisher in stage I–IV were 21/29 (72.4%), 17/22 (77.3%), 16/21 (76.2%), and 6/9 (66.7%) respectively. All P values >0.05, there are no statistical difference between different pathological types and stages in CTC detection rates.
Discussion
Lung cancer is the leading malignancy with the highest morbidity and mortality in the Chinese populations. Despite recent implementation of novel targeted agents, 5-year survival of lung cancer remains at a low rate of 19%. Screening of high risky subjects with LDCT (Low Dose Computed Tomography) can reduce lung cancer-related mortality by 20%, but the high false positive rate of lung nodules leads to morbidities from interventions, unnecessary imaging, and costs (41). Moreover, Rampinelli and coworkers reported that the radiation exposure from LDCT and positron emission tomography-computed tomography (PET-CT) scans for lung cancer is non-negligible (42).
An assay that accurately implements early screening for NSCLC would be greatly welcomed and could improve survival. Ideally, the assay could be safely performed in all patients (i.e., noninvasive), involve minimal or no discomfort. CTC assays may fulfill such roles. Recurrence and metastasis of lung cancer occur via CTCs and seriously affect the survival of patients even after surgery (43). Improving the rate of early screening has significant importance for improving the survival rate of patients, and the key to improving prognosis is to use better approaches for surveillance, continuous detection of CTCs can offer valuable insights.
CTC is extremely rare in peripheral blood, there are about 10 million WBCs and 5 billion red blood cells in 1 mL peripheral blood, but only several CTCs (9). Therefore, it is necessary to enrich the cells before detection. At present, there are two mainly methods to enrich and identify CTCs, based on the principle of morphology and the principle of immune adsorption. The immune adsorption method is the most widely used. It divided into two kinds, positive and negative cell sorting. For positive sorting, EpCAM was used as the marker, captured the CTCs by antigen-antibody specific binding. Representative techniques include CellSearch, CTC chip, etc. (44,45). CellSearch was the most widely used method for CTC detection in the past few years and has been approved by FDA in breast, colorectal and prostate cancer, but the recovery rate of CTCs in lung cancer is only about 20–40% (32,34). For negative sorting, after CD45 antibody specific adsorption, using cell markers identify the CTCs. In addition, isolation by size of epithelial tumour cells (ISET) and some non-commercial morphology-based methods have been employed in CTC detection in small samples of patients with NSCLC (46). The positive rates ranged from 13% to 87% (47,48). The main challenge of CTCs in early NSCLC screening is the lack of stable and highly sensitive methods.
The method used in this study has shown superiority in CTC detection. Peps play key roles in participating in ligand-receptor and protein-protein interactions, since the recognitions are mainly involving in the short Peps at the contact interface (49,50). Compared with the reported CTC analysis results in NSCLC patients, our detection rate (72.8%) is at the upstream level (21). In our study, the number of detected CTCs ranged from 0–8 cells/2 mL, while CellSearch was 0 cell/7.5 mL. The high efficiency result is ascribed to the high density and well-defined orientation of biotin-Pep modified on the magnetic nanoparticle surface with streptavidin functionalization, and also the multivalent interactions because of the Pep assembly structures via disulfide bond between cysteine residues.
For patients with different stages of disease, the CTC number differs. Tanaka and coworkers reported that the positive rates of CTC in lung cancer with stage I–III was only 0–20% (33). In stage I NSCLC, the detection of CTC is more challenging. The detection rates of ISET and a nano-enrichment technique are 49% and 17.4%, respectively (21,51). Our study used a highly sensitive method to detect CTCs of NSCLC patients at the early stage, which has a certain guiding role in clinical practice. The overall detection rate of this method was 72.8%. It is worth mentioning that in stage I, II, III patients, the detection rate reached 72.4%, 77.3%, 76.2% respectively. The results show that magnetic nanoparticles detection technology is expected to be an indicator of early NSCLC screening. Meanwhile, our data shows that there is no linear correlation between the CTC numbers and the clinical stage. Different research results differ, the number of CTCs enriched based on morphological methods is linearly correlated with the clinical stage (52). This result may be related to many reasons, such as epithelial-mesenchymal transition of tumor cells, and a small number of studies (10,53). This small-sample single-center prospective cohort study still has certain limitations. Before TumorFisher can be applied to NSCLC clinically, the next large-sample, multi-center study is still needed.
In summary, the highly efficient CTC detection by TumorFisher was demonstrated both in NSCLC cell line level and patient sample level. The comparison between CTC detection rate TumorFisher (5/7, 71.4%) and CellSearch system (all false negative) showed obviously superiority of our TumorFisher technique. The higher CTC detection rates (66.7–77.3%) were demonstrated for NSCLC patient blood samples ranging from stage I to stage IV, which manifested the possible application of TumorFisher in prognosis, dynamic monitoring of treatment of lung cancer. The distinguished high detection rate in early stage NSCLC patients indicated the feasibility of the TumorFisher technique in early screening of lung cancer. Large scale clinical cohort study could be performed in the future to further confirm the clinical utilities of this technique in not only NSCLC, but also in other lung cancers and even in other cancer types.
Acknowledgments
Funding: This work was supported by Major projects of the Beijing Municipal Science and Technology Commission (Z171100002017013); Special Data Service for Oncology, The National Population and Health Scientific Data Sharing Platform (NCMI-ABD02-201809; NCMI-YF02N-201906); Ministry of Science and Technology of the People’s Republic of China (MOST); Beijing Natural Science Foundation (7182132); Chinese Academy of Medical Sciences (CAMS) Innovation Fund for Medical Sciences (2019-12M-1-001); National Key Research and Development Program of China (2017YFA0205001); Natural Science Foundation of China (21773042).
Footnote
Data Sharing Statement: Available at http://dx.doi.org/10.21037/jtd-20-1026A
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd-20-1026A). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and was approved by the PUMCH ethics committees (JS-1263). All patients enrolled completed the informed consent form.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Goldstraw P, Chansky K, Crowley J, et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2016;11:39-51. [Crossref] [PubMed]
- Wu LX, Li XF, Chen HF, et al. Combined detection of CEA and CA125 for the diagnosis for lung cancer: A meta-analysis. Cell Mol Biol (Noisy-le-grand) 2018;64:67-70. [Crossref] [PubMed]
- National Lung Screening Trial Research Team, Church TR, Black WC, et al. Results of initial low-dose computed tomographic screening for lung cancer. N Engl J Med 2013;368:1980-91. [Crossref] [PubMed]
- Hosseini H, Obradović MMS, Hoffmann M, et al. Early dissemination seeds metastasis in breast cancer. Nature 2016;540:552-8. [Crossref] [PubMed]
- Ashworth TR. A case of cancer in which cells similar to those in the tumours were seen in the blood after death. Aust Med J 1869;14:146-7.
- Hofman V, Bonnetaud C, Ilie MI, et al. Preoperative circulating tumor cell detection using the isolation by size of epithelial tumor cell method for patients with lung cancer is a new prognostic biomarker. Clin Cancer Res 2011;17:827-35. [Crossref] [PubMed]
- Huang X, Gao P, Song Y, et al. Relationship between circulating tumor cells and tumor response in colorectal cancer patients treated with chemotherapy: a meta-analysis. BMC Cancer 2014;14:976. [Crossref] [PubMed]
- Krebs MG, Hou JM, Sloane R, et al. Analysis of circulating tumor cells in patients with non-small cell lung cancer using epithelial marker-dependent and -independent approaches. J Thorac Oncol 2012;7:306-15. [Crossref] [PubMed]
- Krebs MG, Sloane R, Priest L, et al. Evaluation and prognostic significance of circulating tumor cells in patients with non-small-cell lung cancer. J Clin Oncol 2011;29:1556-63. [Crossref] [PubMed]
- Peeters DJ, van Dam PJ, Van den Eynden GG, et al. Detection and prognostic significance of circulating tumour cells in patients with metastatic breast cancer according to immunohistochemical subtypes. Br J Cancer 2014;110:375-83. [Crossref] [PubMed]
- Punnoose EA, Atwal S, Liu W, et al. Evaluation of circulating tumor cells and circulating tumor DNA in non-small cell lung cancer: association with clinical endpoints in a phase II clinical trial of pertuzumab and erlotinib. Clin Cancer Res 2012;18:2391-401. [Crossref] [PubMed]
- Riquet M, Rivera C, Gibault L, et al. Lymphatic spread of lung cancer: anatomical lymph node chains unchained in zones. Rev Pneumol Clin 2014;70:16-25. [Crossref] [PubMed]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011;144:646-74. [Crossref] [PubMed]
- Kapeleris J, Kulasinghe A, Warkiani ME, et al. The Prognostic Role of Circulating Tumor Cells (CTCs) in Lung Cancer. Front Oncol 2018;8:311. [Crossref] [PubMed]
- Jin XR, Zhu LY, Qian K, et al. Circulating tumor cells in early stage lung adenocarcinoma: a case series report and literature review. Oncotarget 2017;8:23130-41. [Crossref] [PubMed]
- Hofman V, Ilie MI, Long E, et al. Detection of circulating tumor cells as a prognostic factor in patients undergoing radical surgery for non-small-cell lung carcinoma: comparison of the efficacy of the CellSearch Assay and the isolation by size of epithelial tumor cell method. Int J Cancer 2011;129:1651-60. [Crossref] [PubMed]
- Hanssen A, Loges S, Pantel K, et al. Detection of Circulating Tumor Cells in Non-Small Cell Lung Cancer. Front Oncol 2015;5:207. [Crossref] [PubMed]
- Hou JM, Greystoke A, Lancashire L, et al. Evaluation of circulating tumor cells and serological cell death biomarkers in small cell lung cancer patients undergoing chemotherapy. Am J Pathol 2009;175:808-16. [Crossref] [PubMed]
- Horton CE, Kamal M, Leslie M, et al. Circulating Tumor Cells Accurately Predicting Progressive Disease After Treatment in a Patient with Non-small Cell Lung Cancer Showing Response on Scans. Anticancer Res 2018;38:1073-6. [PubMed]
- Wei T, Zhu D, Yang Y, et al. The application of nano-enrichment in CTC detection and the clinical significance of CTCs in non-small cell lung cancer (NSCLC) treatment. PloS one 2019;14:e0219129. [Crossref] [PubMed]
- Bidard FC, Kiavue N, Ychou M, et al. Circulating Tumor Cells and Circulating Tumor DNA Detection in Potentially Resectable Metastatic Colorectal Cancer: A Prospective Ancillary Study to the Unicancer Prodige-14 Trial. Cells 2019;8:516. [Crossref] [PubMed]
- Goldkorn A, Ely B, Quinn DI, et al. Circulating tumor cell counts are prognostic of overall survival in SWOG S0421: a phase III trial of docetaxel with or without atrasentan for metastatic castration-resistant prostate cancer. J Clin Oncol 2014;32:1136-42. [Crossref] [PubMed]
- Krebs MG, Renehan AG, Backen A, et al. Circulating Tumor Cell Enumeration in a Phase II Trial of a Four-Drug Regimen in Advanced Colorectal Cancer. Clin Colorectal Cancer 2015;14:115-22.e1-2.
- Larsson AM, Jansson S, Bendahl PO, et al. Longitudinal enumeration and cluster evaluation of circulating tumor cells improve prognostication for patients with newly diagnosed metastatic breast cancer in a prospective observational trial. Breast Cancer Res 2018;20:48. [Crossref] [PubMed]
- Li Y, Gong J, Zhang Q, et al. Dynamic monitoring of circulating tumour cells to evaluate therapeutic efficacy in advanced gastric cancer. Br J Cancer 2016;114:138-45. [Crossref] [PubMed]
- Meyer CP, Pantel K, Tennstedt P, et al. Limited prognostic value of preoperative circulating tumor cells for early biochemical recurrence in patients with localized prostate cancer. Urol Oncol 2016;34:235.e11-6. [Crossref] [PubMed]
- Rack B, Schindlbeck C, Jückstock J, et al. Circulating tumor cells predict survival in early average-to-high risk breast cancer patients. J Natl Cancer Inst 2014;106:dju066. Erratum in: J Natl Cancer Inst. 2014 Sep;106(9):doi/10.1093/jnci/dju273. [Crossref] [PubMed]
- Tamminga M, de Wit S, Hiltermann TJN, et al. Circulating tumor cells in advanced non-small cell lung cancer patients are associated with worse tumor response to checkpoint inhibitors. J Immunother Cancer 2019;7:173. [Crossref] [PubMed]
- Hirose T, Murata Y, Oki Y, et al. Relationship of circulating tumor cells to the effectiveness of cytotoxic chemotherapy in patients with metastatic non-small-cell lung cancer. Oncol Res 2012;20:131-7. [Crossref] [PubMed]
- Isobe K, Hata Y, Kobayashi K, et al. Clinical significance of circulating tumor cells and free DNA in non-small cell lung cancer. Anticancer Res 2012;32:3339-44. [PubMed]
- Went PT, Lugli A, Meier S, et al. Frequent EpCAM protein expression in human carcinomas. Hum Pathol 2004;35:122-8. [Crossref] [PubMed]
- Tanaka F, Yoneda K, Kondo N, et al. Circulating tumor cell as a diagnostic marker in primary lung cancer. Clin Cancer Res 2009;15:6980-6. [Crossref] [PubMed]
- Bai L, Du Y, Peng J, et al. Peptide-based isolation of circulating tumor cells by magnetic nanoparticles. J Mater Chem B 2014;2:4080-8. [Crossref] [PubMed]
- Yue C, Jiang Y, Li P, et al. Dynamic change of PD-L1 expression on circulating tumor cells in advanced solid tumor patients undergoing PD-1 blockade therapy. Oncoimmunology 2018;7:e1438111. [Crossref] [PubMed]
- Liu XR, Shao B, Peng JX, et al. Identification of high independent prognostic value of nanotechnology based circulating tumor cell enumeration in first-line chemotherapy for metastatic breast cancer patients. Breast 2017;32:119-25. [Crossref] [PubMed]
- Travis WD, Dacic S, Wistuba I, et al. IASLC Multidisciplinary Recommendations for Pathologic Assessment of Lung Cancer Resection Specimens After Neoadjuvant Therapy. J Thorac Oncol 2020;15:709-40. [Crossref] [PubMed]
- Du H, Hu X, Duan H, et al. Principles of Inter-Amino-Acid Recognition Revealed by Binding Energies between Homogeneous Oligopeptides. ACS Cent Sci 2019;5:97-108. [Crossref] [PubMed]
- Wang C, Mao X, Yang A, et al. Determination of relative binding affinities of labeling molecules with amino acids by using scanning tunneling microscopy. Chem Commun (Camb) 2011;47:10638-40. [Crossref] [PubMed]
- Dementeva N, Kokova D, Mayboroda OA. Current Methods of the Circulating Tumor Cells (CTC) Analysis: A Brief Overview. Curr Pharm Des 2017;23:4726-8. [Crossref] [PubMed]
- Aberle DR, Adams AM, Berg CD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011;365:395-409. [Crossref] [PubMed]
- Rampinelli C, De Marco P, Origgi D, et al. Exposure to low dose computed tomography for lung cancer screening and risk of cancer: secondary analysis of trial data and risk-benefit analysis. BMJ 2017;356:j347. [Crossref] [PubMed]
- Manjunath Y, Upparahalli SV, Suvilesh KN, et al. Circulating tumor cell clusters are a potential biomarker for detection of non-small cell lung cancer. Lung cancer 2019;134:147-50. [Crossref] [PubMed]
- Allard WJ, Matera J, Miller MC, et al. Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clin Cancer Res 2004;10:6897-904. [Crossref] [PubMed]
- Nagrath S, Sequist LV, Maheswaran S, et al. Isolation of rare circulating tumour cells in cancer patients by microchip technology. Nature 2007;450:1235-9. [Crossref] [PubMed]
- Vona G, Sabile A, Louha M, et al. Isolation by size of epithelial tumor cells: a new method for the immunomorphological and molecular characterization of circulatingtumor cells. Am J Pathol 2000;156:57-63. [Crossref] [PubMed]
- Lecharpentier A, Vielh P, Perez-Moreno P, et al. Detection of circulating tumour cells with a hybrid (epithelial/mesenchymal) phenotype in patients with metastatic non-small cell lung cancer. Br J Cancer 2011;105:1338-41. [Crossref] [PubMed]
- Zhang Z, Xiao Y, Zhao J, et al. Relationship between circulating tumour cell count and prognosis following chemotherapy in patients with advanced non-small-cell lung cancer. Respirology 2016;21:519-25. [Crossref] [PubMed]
- Sadatmousavi P, Soltani M, Nazarian R, et al. Self-assembling peptides: potential role in tumor targeting. Curr Pharm Biotechnol 2011;12:1089-100. [Crossref] [PubMed]
- Ueno S, Yoshida S, Mondal A, et al. In vitro selection of a peptide antagonist of growth hormone secretagogue receptor using cDNA display. Proc Natl Acad Sci U S A 2012;109:11121-6. [Crossref] [PubMed]
- Hofman V, Long E, Ilie M, et al. Morphological analysis of circulating tumour cells in patients undergoing surgery for non-small cell lung carcinoma using the isolation by size of epithelial tumour cell (ISET) method. Cytopathology 2012;23:30-8. [Crossref] [PubMed]
- Wan JW, Gao MZ, Hu RJ, et al. A preliminary study on the relationship between circulating tumor cells count and clinical features in patients with non-small cell lung cancer. Ann Transl Med 2015;3:352. [PubMed]
- Pastushenko I, Brisebarre A, Sifrim A, et al. Identification of the tumour transition states occurring during EMT. Nature 2018;556:463-8. [Crossref] [PubMed]


