
The length of the left superior pulmonary vein stump after left upper lobectomy depends on its position to the left atrial appendage
Introduction
Although rare, cerebral infarction remains a concern after surgery, with an occurrence rate of 0.6–1% after general thoracic surgery in Japan (1-3). After lung lobectomy, a longer length of the left superior pulmonary vein (LSPV) stump may increase the risk for postoperative cerebral infarction (4). Recent studies demonstrated that the length of the pulmonary vein (PV) stump is generally longer after left upper lobectomy (LUL) than after other lobectomies (5), and that thrombus formation, which can cause a cardiogenic stroke, is more likely with a longer LSPV stump after LUL (4,5). Moreover, the length of the LSPV stump after LUL may also vary across patients, due to anatomical differences in the relationship between the LSPV and the left atrial appendage (LAA). In this study, we hypothesized that the length of the LSPV stump after LUL would vary as a function of the anatomical relationship between the LSPV and the LAA. Therefore, our aim was to investigate if the length of the LSPV stump was related to the anatomical position of the LSPV in relation to LAA. We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/jtd-20-1170).
Methods
Patients
We retrospectively reviewed 91 patients who underwent LUL at our institution, between January 2014 and March 2018. Two patients presenting with an intra-cardium division of the LSPV and three patients with a common trunk of the left PV were excluded. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The approval from the Institutional Review Board of this study was waived because of the retrospective character of the study.
Two thoracic surgeons analyzed the computed tomography (CT) and patients were classified into two groups, based on the anatomical relationship between the LSPV and LAA: an antero-superior pattern and a postero-inferior pattern. The clinicopathological data were obtained from the medical charts. The pathological staging was based on the 7th edition of the Union Internationale Contra le Cancer (UICC) staging system on tumor-node-metastasis (TNM) classification.
Operative procedure
Video-assisted thoracoscopic surgery (VATS) was performed for all cases except those with large tumor size, chest wall invasion, bronchial invasion, or pulmonary vessel invasion. The PV during lobectomy was generally divided in the extra-pericardial space, using linear staplers (GIA Stapling System, Ethicon, Dublin, Ireland and Endo GIA, Covidien, Dublin, Ireland), except in a few cases where an intra-pericardial procedure was needed. The dissection line of the LSPV was as proximal to the atrium as possible.
Anatomical location of the LSPV
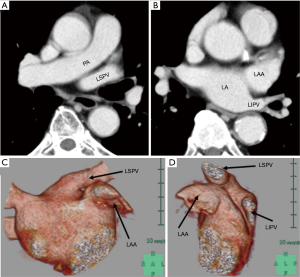
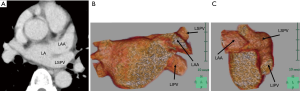
The anatomical location of the LSPV was classified into two types, according to the position of the LSPV relative to the LAA on pre-operative CT images, as indicated above: an antero-superior pattern and a postero-inferior pattern. In the antero-superior pattern, the inlet of the LSPV is located antero-superior to the LAA (Figure 1A), with the LAA observed at the level of the inlet of the left inferior pulmonary vein (LIPV) on axial CT images (Figure 1B). This antero-superior position of the LSPV inlet relative to the LAA was confirmed on three-dimensional reconstruction of the PV, LAA, and left atrium (LA) from pre-operative contrast-enhanced CT images (CE-CT) (Figure 1C,D). In the postero-inferior pattern, the LSVP courses posterior to the lower part of LAA, with the inlet of the LSPV located at the level of the LAA on axial pre-operative CT images (Figure 2A). The LSPV can be seen postero-inferior to the LAA on three-dimensional reconstruction of the PV, LAA, and LA from pre-operative CE-CT (Figure 2B,C).
Measurement of the length of superior PV stump after LUL
Plain CT or CE-CT was performed 1–3 months after surgery. The length of the LSPV stump after LUL was defined as the linear distance from the inlet of the LSPV to the stump, measured on postoperative CT by making oblique multi-planar reconstruction (MPR) formatted images, using CT workstation tools (Ziostation2, Ziosoft Inc., Tokyo, Japan) (Figure 3) (6).

Statistical analysis
Analysis of the difference in the length of the LSPV stump after LUL between the antero-superior and postero-inferior pattern was performed using a t-test. This statistical analysis was performed using the StatMate IV software program (Advanced Technology for Medicine and Science Co., Ltd., Tokyo, Japan). A P value of less than 0.05 was regarded as significant.
Results
Eighty-five patients met our inclusion criteria for enrollment, 58 men and 27 women, with a mean age of 72 (range, 17–85) years. Preoperative comorbidities consisted 39 cases of hypertension, 17 cases of diabetes mellitus, 15 cases of chronic obstructive pulmonary diseases, 14 cases of ischemic heart diseases, 9 arrhythmias and 7 cerebral vascular diseases. The operative approaches were 62 VATS, and 23 open thoracotomies. The median days of chest drainage were 5 (range, 2–19) days. Median hospital length of stay was 8 (range, 4–26) days. Postoperative complications consisted 5 cases of prolonged air leakage, 4 cases of atrial fibrillation, and a cerebral infarction. There were 80 cases of primary lung cancer, 3 of metastatic lung tumors, and 2 of benign tumors. Of the primary lung cancers, there were 51 adenocarcinomas, 20 squamous cell carcinomas, 3 of small cell carcinomas, 3 of pleomorphic carcinomas, 2 of adenosquamous cell carcinomas, and 1 of large cell carcinoma. Pathological stage of the primary lung carcinomas consisted of 55 cases of Stage I, 11 of Stage II, and 14 of Stage III.
Of 85 cases, 49 (57.6%) patients were classified in the antero-superior pattern group and 36 (42.4%) into the postero-inferior pattern group. In male and female, 34 (58.6%) and 15 (55.6%) patients were classified in the antero-superior pattern group and 24 (41.4%) and 12 (44.4%) into the postero-inferior pattern group, respectively. Postoperatively, plain CT imaging was performed in 57 patients and CE-CT in 28. Among the 28 patients who underwent postoperative CE-CT, no incidence of thrombus development in the LSPV stump was observed. Cerebral infarction occurred in one patient in the antero-superior group, whose length of the LSPV stump was 31 mm, on postoperative day 4, with no evidence of thrombus formation on CE-CT.
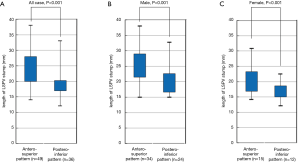
The mean length of the LSPV stump after LUL, overall, male, and female, were 21.9 (range, 15–38), 23.2 (range, 15–38), and 19.2 (12–31) mm, respectively. In all cases, the stump was significantly longer in the antero-superior (mean 24.2 mm, range 14–38 mm) than the postero-inferior (mean 18.9 mm, range 12–33 mm) group (P<0.001). In male patients, the stump was significantly longer in the antero-superior (mean 25.4 mm, range 15–38 mm) than the postero-inferior (mean 19.9 mm, range 15–33 mm) group (P<0.001). In female patients, the stump was significantly longer in the antero-superior (mean 21.3 mm, range 14–31 mm) than the postero-inferior (mean 16.8 mm, range 12–22 mm) group (P<0.001) (Figure 4).
Discussion
In the present study, we clarified that the length of LSPV stump, estimated on postoperative CT images, was closely related to the anatomical relationship between the LSPV and LAA on pre-operative CT images. Specifically, the length of the LSPV stump was significantly longer in patients with an antero-superior anatomical pattern than a postero-inferior pattern. This longer length of the LSPV stump after LUL in the antero-superior pattern results from the location of the left hilum posterior to the LAA. The length of the PV stump after lobectomy was nearly equal to the length of the intra-pericardial PV, as the PV was generally dissected to be as short as possible, using linear staplers at the hilum. Overall, our findings support a positive association between the anatomical relationship between the LSPV and LAA and the length of the intra-pericardial LSPV stump, with the stump being longer for the antero-superior than the postero-inferior pattern after LUL.
A previous study identified an increased prevalence of thrombosis development with longer PV stumps (7). In a short PV stump, blood would flow from the LA throughout the PV stump, while in a longer PV stump, turbulent flow or stasis might result as blood flow from the LA likely does not spread throughout the PV stump (5). Our finding that the anatomical location of LSPV was associated with the length of the LSPV stump could be used for a more effective and efficient peri-operative management, such as administration of anticoagulant therapy.
The limitations of our study need to be acknowledged. Firstly, as the study was a retrospective study, selection bias was neglected. Secondly, we were unable to conclude whether long LSPV stumps are related to thrombus formation causing cardiogenic stroke, because only 28 patients underwent postoperative CE-CT. Thirdly, our sample size with small and only one patient had stroke postoperatively. We could not make occurrence of stroke after left lower lobectomy as an outcome of this study. Therefore, we could not clarify the relation between the anatomical relationship between the LSVP and LAA and the risk of stroke. A future study is needed to confirm or refute findings.
Conclusions
The anatomical location of the LSPV relative to the LAA is associated with the length of the LSPV stump after LUL. Based on previous studies that have reported an increased risk for cerebral infarct with a longer LSPV stump length after LUL, thoracic surgeons should analyze the relationship between the anatomical location of the LSPV and the LAA on pre-operative CT.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement reporting checklist. Available at http://dx.doi.org/10.21037/jtd-20-1170
Data Sharing Statement: Available at http://dx.doi.org/10.21037/jtd-20-1170
Peer Review File: Available at http://dx.doi.org/10.21037/jtd-20-1170
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd-20-1170). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The approval from the Institutional Review Board of this study was waived because of the retrospective character of the study. Informed consent was waived due to the retrospective nature of the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Hattori A, Takamochi K, Kitamura Y, et al. Risk factor analysis of cerebral infarction and clinicopathological characteristics of left upper pulmonary vein stump thrombus after lobectomy. Gen Thorac Cardiovasc Surg 2019;67:247-53. [Crossref] [PubMed]
- Matsumoto K, Sato S, Okumura M, et al. Frequency of cerebral infarction after pulmonary resection: a multicenter, retrospective study in Japan. Surg Today 2018;48:571-2. [Crossref] [PubMed]
- Yamamoto T, Suzuki H, Nagato K, et al. Is left upper lobectomy for lung cancer a risk factor for cerebral infarction? Surg Today 2016;46:780-4. [Crossref] [PubMed]
- Ohtaka K, Hida Y, Kaga K, et al. Thrombosis in the pulmonary vein stump after left upper lobectomy as a possible cause of cerebral infarction. Ann Thorac Surg 2013;95:1924-8. [Crossref] [PubMed]
- Ohtaka K, Hida Y, Kaga K, et al. Left upper lobectomy can be a risk factor for thrombosis in the pulmonary vein stump. J Cardiothorac Surg 2014;9:5. [Crossref] [PubMed]
- Wannasopha Y, Oilmungmool N, Euathrongchit J. Anatomical variations of pulmonary venous drainage in Thai people: multidetector CT study. Biomed Imaging Interv J 2012;8:e4. [PubMed]
- Kwek BH, Wittram C. Postpneumonectomy pulmonary artery stump thrombosis: CT features and imaging follow-up. Radiology 2005;237:338-41. [Crossref] [PubMed]


