Comparison of the progression-free survival between robot-assisted thymectomy and video-assisted thymectomy for thymic epithelial tumors: a propensity score matching study
Introduction
Thymic epithelial tumor is the most common primary tumor in the anterior mediastinum of adults. Minimally invasive surgical approaches for thymectomy have gained popularity for patients with thymic epithelial tumors. Several reports demonstrated that that video-assisted thoracoscopic thymectomy (VATT) has improved short-term outcomes comparing to open thymectomy. Compared with open thymectomy, minimally invasive surgical has been associated with lower operative blood loss, shorter hospital stays, shorter length of stay in the intensive care unit, improved postoperative pulmonary function, decreased postoperative pain (1-4). Robot-assisted thoracoscopic thymectomy (RATT), with da Vinci Surgical Robotic System (Intuitive Surgical, Inc, Sunnyvale, CA, USA), was later introduced, and it became increasingly popular with its benefits over VATT (5-8). This technology has been performed in many thoracic surgery procedures, with clear evidence that the robotic system provides its best advantage when operating in tiny and difficult-to-reach anatomic regions such as the mediastinum (9,10). Therefore, mediastinal diseases were selected electively by surgeons for clinical research using the da Vinci robot.
There have been a variety of studies comparing the results of video-assisted thoracoscopic surgery (VATS) for anterior mediastinal masses with a sternotomy approach (11-13), however, there are few studies that compared the clinical outcomes in patients undergoing RATT with those undergoing VATT. Meanwhile, no study reported the impact of the use of RATT on survival time. Therefore, whether RATT has the superiorities over VATT in short-term outcomes and progression-free survival (PFS) remains controversial. In clinical practice, we found it is easier to remove the superior poles and upper border of thymus and lymph nodes by using robotic technique comparing to thoracoscopic technique. Meanwhile, the robotic system could present more elaborate dissection of thymic epithelial tumors compared with thoracoscopic technique, which could better maintain the membrane integrity. Therefore, we expected longer PFS in the robot group due to more radical dissection of the peripheral tissue and lymph nodes and preferably maintaining the membrane integrity. The aim of this study was to evaluate the PFS and short-term clinical outcomes in patients undergoing RATT by comparing the matched two groups after performing propensity score analysis. We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/jtd-20-1065).
Methods
We retrospectively reviewed the clinical data of 295 patients diagnosed with thymic epithelial tumors between December 1, 2009 and December 1, 2014 at the Department of Thoracic Surgery in Nanjing Jinling Hospital. The diagnoses and clinical information were collected by consulting medical records of the patients. The selection criteria of minimally invasive approach in our institution are listed as follows: (I) the size of thymic epithelial tumor is shorter than 10 cm; (II) the macro vessels are not invaded by the thymic epithelial tumor; (III) the tumor invasion in lung or pericardium is resectable. No artificial intervention or different treatment methods were deliberately implied for patients to obtain a certain group of data since this is a retrospective study. All patients were treated according to the actual clinical needs. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and the Harmonized Tripartite Guideline for Good Clinical Practice from the International Conference on Harmonization. This study was approved by the Ethics Committee of Jingling Hospital. The ethics committee waived the need for informed consents from those patients since our study was a retrospective cohort analysis and analyzed anonymously.
Clinical outcomes
Patients’ characteristics included age, gender and myasthenia gravis (MG). Meanwhile, tumor-associated data, including tumor size, organization histologic classification, original Masaoka stages, adjuvant therapy and chemotherapy were also collected. Surgical data consisted of surgical time (from skin incision to closure) and blood loss. The primary endpoint of this study was PFS. And the secondary endpoints were the early clinical outcomes of surgery including duration of chest tube insertion, median volume of drainage, postoperative hospital stay and postoperative complications. The short-term endpoints were selected according to literature review. We compared the perioperative outcomes using all thymic epithelial tumors’ data. And PFS were estimated in only thymoma cases. If data were missing, an imputation method was used to integrate the data.
Follow-up
All patients were followed up every 3 months for the first 2 years, then every 6 months thereafter. Our follow-ups were conducted on telephone, through outpatient department visit, or home visit and the last follow-up was implemented in December 1, 2019. The image examination during the follow-up included chest computerized tomography (CT) scan. Whenever recurrence was suspected, we attempted to obtain histological or unequivocal radiological proof. Recurrence was classified in to loco-regional recurrence and distal metastasis. PFS was defined as the time form the data of operation to recurrence.
Surgical method
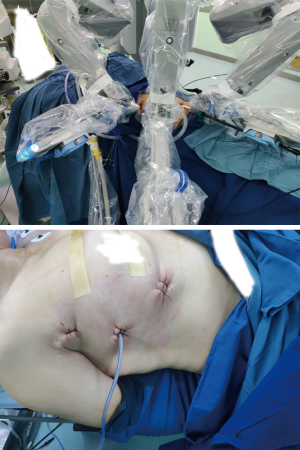
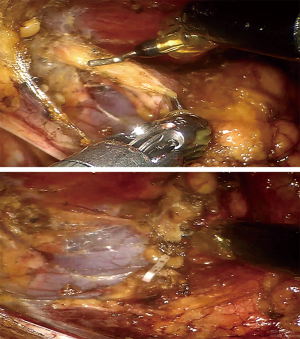
Because we offered two choices of minimally invasive thymectomy (RATT and VATT) to those patients, the surgical methods were determined by patients’ willing after exhaustively introducing the two surgical approaches to patients. Both groups had the same management and patient selection protocols. Contrast-enhanced chest CT was performed in all patients before operations to identify the tumors’ relationships with surrounding tissues. In RATT, the patient is positioned supine on the surgical table with the right side elevated at 30º. Three-arm technique is used in operation and the camera trocar was first inserted at the fifth intercostal space (ICS), along the anterior axillary line. Another trocar for grasping and dissecting arm was inserted at the second or third ICS, along the anterior axillary line. Then, the third trocar for an ultrasonic device was inserted at the fifth or sixth ICS, along the midclavicular line (Figure 1). The surgery was performed from a phrenic nerve to the other, with maximum removal of superior poles by gradual pulling down and resection. The upper border is the inferior thyroid plexus. All patients received anterior mediastinal fat dissection, during which adipose tissue was removed (Figure 2). In VATT, artificial pneumothorax was used. The patient is positioned supine on the surgical table with the right side elevated at 30–40º. The camera trocar was first inserted at the sixth ICS, along the middle axillary line. The other two trocars were inserted at the fourth or seventh ICS respectively, both along the anterior axillary line. The surgical procedure of VATT was same to that of RATT.
Statistical analysis
Categorical variables were expressed as frequencies and compared using the Fisher exact test or Chi-square tests. Continuous variables were expressed as mean ± standard deviation and compared using the independent-sample Student’s t-test or the Mann-Whitney non-parametric U-test. Survival time was calculated by the Kaplan-Meier analysis, and the log-rank test was used to explore the association between variables and survival time. The Cox’s hazard regression model was applied to investigate independent prognostic factors by univariate analysis and multivariate analysis. In order to balance baseline characteristics between the two groups, propensity-score matched (PSM) analysis was performed using R 3.6.1. The propensity scores were calculated with covariates including age, gender, organization histologic classification, original Masaoka stages, MG and adjuvant therapy. Cases from the two groups were matched at a ratio of 1:1 by using the nearest-neighbor method with a caliper width of 0.05. The effect of treatment on the outcomes was done in a manner that accounts for the matched nature of the propensity score-matched sample. The statistical significance of the effect of exposure on continuous outcomes was assessed by a paired t-test or the Wilcoxon signed rank test. Proportions were compared by the McNemar’s test for correlated binary proportions, or extensions thereof for categorical variables with more than two levels.
Results
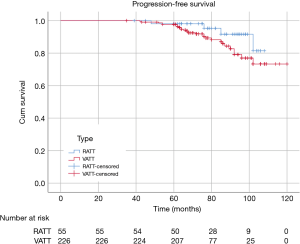
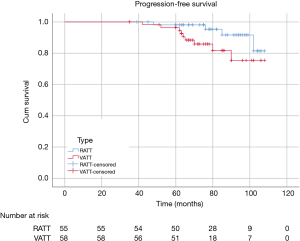
There were 60 patients in RATT group and 235 in VATT group. The perioperative and investigative profile of the entire study population is presented in Table 1. And the short-term clinical outcomes of two groups were compared in Table 2. The surgical time (P=0.01) between two groups were significantly different. There were 2 additional resections in RATT group and 4 in VATT group. Only one case in RATT group was converted to open surgery while 8 cases in VATT group were converted to open operation. In-hospital mortality happened in 1 case in VATT group. The PFS was compared in Figure 3. The median follow-up time was about 75.5 months, with the longest follow-up time of 120 months. RATT was first introduced on December 12, 2011. Loco-regional recurrence and distal metastasis were detected in 4 patients with thymoma and 1 patient with thymic carcinoma in RATT group and in 25 patients with thymoma and 3 patients with thymic carcinoma in VATT group before matching. However, no lymph node recurrence or distal metastasis was identified in type A, AB, or B1 thymomas in RATT group. Only 2 patients with type B1 thymoma in VATT group was detected with loco-regional recurrence and no lymph node recurrence or distal metastasis was identified in type A or AB thymomas. PFS were estimated in only thymoma cases. There was no significant difference in PFS between RATT group (n=55) and VATT group (n=226) before matching (5-year PFS rate: 81.5% and 73.3%, respectively; log-rank P=0.146).
Full table

Full table
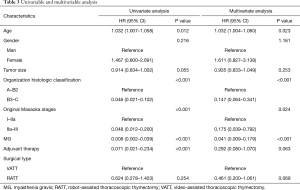
For prognosis, both univariate and multivariate analyses were applied (Table 3). Cox regression model was calculated according to age, gender, tumor size, organization histologic classification, original Masaoka stages, presence of MG and adjuvant therapy as independent variables. According to the results of univariate analyses, age (HR: 1.032; 95% CI: 1.007–0.058, P=0.012), organization histologic classification (HR: 0.046; 95% CI: 0.021–0.102, P<0.001), original Masaoka stages (HR: 0.048; 95% CI: 0.012–0.200, P<0.001), presence of MG (HR: 0.008; 95% CI: 0.002–0.039, P<0.001) and adjuvant therapy (HR: 0.071; 95% CI: 0.021–0.234, P<0.001) had significant influence on PFS. As to the results of multivariate analyses, age (HR: 1.032; 95% CI: 1.004–0.060, P=0.023), organization histologic classification (HR: 0.147; 95% CI: 0.064–0.341, P<0.001), original Masaoka stages (HR: 0.175; 95% CI: 0.039–0.792, P=0.024) and presence of MG (HR: 0.041; 95% CI: 0.009–0.179, P<0.001) were the independent risk factor of PFS.
Full table
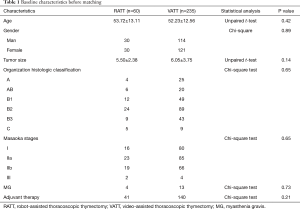
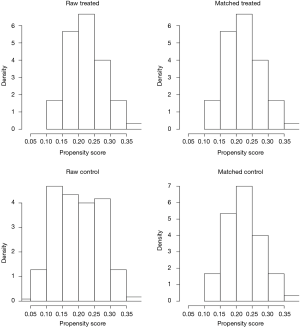
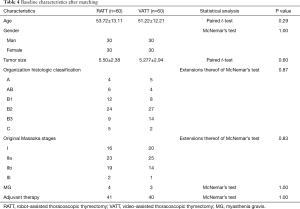
To investigate the actual impact of surgical type on PFS of patients with thymic epithelial tumors, PSM analysis was conducted by matching the following covariates: age, gender, tumor size, organization histologic classification, original Masaoka stages, presence of MG and adjuvant therapy. A histogram showing the distribution of propensity scores was in Figure 4. A total of 60 pairs were matched, and the baseline characteristics of the two groups are shown and compared in Table 4. All these baseline characteristics between RATT group and VATT group were comparable and there was no significant difference between two groups in all variates. And the short-term clinical outcomes were compared between two groups in Table 5. The surgical time in RATT groups was significant shorter than that in VATT groups (P=0.042). However, there was no significant difference in postoperative complications, duration of chest tube insertion, the median volume of drainage (in first 24 hours) or postoperative hospital stay. Loco-regional recurrence and distal metastasis were detected in 4 patients with thymoma and 1 patient with thymic carcinoma in RATT group and in 8 patients with thymoma and 1 patient with thymic carcinoma in VATT group after matching. PFS were estimated in only thymoma cases. The PFS in RATT group (n=55) intended to be longer than that in VATT group (n=58) after matching (5-year PFS rate: 81.5% and 75.4%, respectively; log-rank P=0.095) (Figure 5).
Full table

Full table
Discussion
Thymic epithelial tumors is a rare epithelia neoplasm of the thymus gland, and it is the most common tumor of the anterior superior mediastinum, comprising 20–30% of mediastinal masses in adults (14). In the early 1990s, Roviaro (15) reported on VATT. Comparing VATS and sternotomy for the treatment of MG, it has been shown that VATS is typically associated with reduced blood loss and a shorter operative time and hospital stay, while VATS may also lower the incidence rate of post-operative complications by reducing tissue damage, post-operative pain, and the risk of infection (16,17). Berman et al. (18) reported their first robot-assisted surgery of an anterior mediastinal mass with the Zeus robotic surgical system (Computer Motion, Inc., USA), which lacked three-dimensional (3D) technology. From that time, the role of robot-assisted surgery in the treatment of thymic epithelial tumors has been growing. There has been concern over video-assisted thoracoscopic resection of malignant mediastinal tumors (19-21). With the advantages of minimal invasiveness and rapid recovery, VATS has been adopted more often in recent years to treat early-stage thymic epithelial tumors. When comparing RATS (the newest MG surgical therapy) and sternotomy for the treatment of MG, it has been demonstrated that RATS has favorable outcomes in terms of surgical complications and hospital stay (3,22). With the development of latest robotic technology, 3D vision with multiarticulated arm movement, which reduces surgeons’ tremor, enables better visualization and meticulous dissection of the anterior mediastinal structures. Several studies demonstrated that RATT have the superiorities over VATT in anterior mediastinal masses.
Weksler et al. (23) reported less blood loss, shorter hospital stay and less complications in the robot group than that in the thymectomy, but this study was an unmatched and retrospective study with 15 robotic cases. The study of Cakar et al. (22) enrolled 19 patients. The results showed longer operation time and shorter hospital stay with good neurological outcomes from robotic thymectomies in non-thymomatous MG comparing to open thymectomy. Balduyck et al. (24) reported that decreased quality-of-life scores after surgery in the robotic group approximated baseline preoperative values faster than that in the sternotomy group. Meanwhile, the high burden of decreased physical functioning reported after sternotomy is not seen after a da Vinci robotic-assisted resection. A propensity score matching study of Seong et al. reported that comparing to the patients underwent open thymectomy, less 24-hour tube drainage, less hemoglobin loss, less hematocrit decrease, shorter chest tube days and shorter length of hospital stay were seen in the patients in robot group. The study of Qian et al. (14) is the first research to compare three approaches (median sternotomy, video-assisted thoracic surgery and robot-assisted thoracic surgery) for the treatment of thymic epithelial tumors. Their results revealed intra-operative blood loss volume, mean post-operative pleural drainage duration, pleural drainage volume, and the mean duration of hospital stay show more favorable results in the RATT group versus the VATT or median sternotomy group (all with P<0.05). However, none of the aforementioned studies investigated the survival time in patients undergoing RATT. Recently, O’Sullivan et al. conducted a systematic review and meta-analysis to compare the short-term clinical outcomes between VATT and RATT. The results showed that there was no significant difference between two techniques in surgical time, estimated blood loss, length of hospital stay, conversions to open, intraoperative complications, postoperative complications or mortality (25).
The oncologic outcome in patients underwent RATT in terms of overall survival and tumor-related survival is promising, but a longer follow-up survival analysis is needed to investigate whether robotic thymectomy could be considered as a standard approach. And there was no study comparing the 5-year PFS between RATT and VATT. In our study, PFS and short-term outcomes were both compared between patients in RATT and VATT. The results revealed the same early clinical outcomes between RATT group and VATT group in terms of the duration of chest tube, the median volume of drainage, the incidence of postoperative complications and in-hospital stay. Interestingly, the surgical time in RATT is significantly shorter than that in VATT, attributing to the tremor filtration, 3D visualization, ten-times-enlarged image and seven-degree freedom of its dexterity endowrists in RATT, which enabled the surgeon to operate in a stable and comfortable environment. Only one case in RATT group was converted to open operation. The results demonstrate that RATT for thymic epithelial tumors is easily adaptable and does not require many cases for the learning curve.
As to PFS, type B2 and B3 thymomas showed significantly higher rate of loco-regional recurrence and distal metastasis than type A, AB, and B1 thymomas in this study. Loco-regional recurrence was only detected in 2 patients in VATT group with B1 thymoma. And no distal metastasis was identified in type A, AB, and B1 thymomas in this study. In clinical practice, we found it is easier to remove the superior poles and upper border of thymus by using da Vinci Surgical Robotic System comparing to thoracoscope. Meanwhile, RATT is found to be easier to remove the superior poles and upper border of thymus by using da Vinci robot system comparing to thoracoscope. Furthermore, RATT showed superiority in the aspect of the dissection of anterior mediastinal fat and adipose tissue over VATT. Therefore, we expected a longer PFS in the robot group due to its more radical dissection of the peripheral tissue, the remnant of which is regarded as the risk factor of the recurrence of thymoma (11). Meanwhile, the robotic system could present more elaborate dissection of tumor compared with thoracoscopic technique, which could better maintain the membrane integrity, the destruction of which could result in the dissemination of thymic epithelial tumor. In this study, PFS were estimated in only thymoma cases. The results showed that the PFS in RATT group (n=55) intended to be longer than that in VATT group (n=58) after matching (5-year PFS rate: 81.5% and 75.4%, respectively; log-rank P=0.095). Lymph node dissection could be another potential prognostic factor of PFS after surgery. However, lymph node dissection after thymectomy was not implied in our institution between 2009 and 2014. In patients with MG, approach from bilateral sides was performed in VATT group, while unilateral-side approach was sufficient for thoroughly resecting peripheral tissue of thymic epithelial tumor in RATT. Therefore, we believed RATT could be considered as a standard approach for treatment of thymic epithelial tumors.
Limitation
There are some limitations in our study. First, this is a single-center retrospective study, which could have suffered from the retrospective nature of our study. Second, although propensity score matching was used in this study, the small sample size in our study could still result in selection bias. Third, lymph node dissection, which is the advantage of robotic technique, was not implied in our institution between 2009 and 2014.
Conclusions
RATT has the superiorities over VATT on short-term outcomes due to enabling surgeons to operate in a stable and comfortable environment. Meanwhile, RATT yielded a longer PFS compared with VATT, although the difference was not significant. Therefore, RATT could be considered as a standard approach for the treatment of thymic epithelial tumors.
Acknowledgments
We thank all the members of the Department of Cardiothoracic Surgery in our hospital who participated in this research. We also thank Xiao-Kun Li for his advice on statistical analysis.
Funding: This work was supported by the National Natural Science Foundation of China (No. 81172032) and the Natural Science Foundation of Jiangsu Province (BK20181239).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/jtd-20-1065
Data Sharing Statement: Available at http://dx.doi.org/10.21037/jtd-20-1065
Peer Review File: Available at http://dx.doi.org/10.21037/jtd-20-1065
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd-20-1065). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and the Harmonized Tripartite Guideline for Good Clinical Practice from the International Conference on Harmonization. This study was reviewed and approved by the Institutional Review Board of the Jingling Hospital (approval number 2011NZKY-030-01). All patients enrolled completed the informed consent form. The study outcomes will not affect the future patient management. This study is based on data retrieved from a hospital medical record system. All personal data have been protected and secured according to current national and international laws.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Burt BM, Yao X, Shrager J, et al. Determinants of complete resection of thymoma by minimally invasive and open thymectomy: analysis of an international registry. J Thorac Oncol 2017;12:129-36. [Crossref] [PubMed]
- Jurado J, Javidfar J, Newmark A, et al. Minimally invasive thymectomy and open thymectomy: outcome analysis of 263 patients. Ann Thorac Surg 2012;94:974-81; discussion 981-2. [Crossref] [PubMed]
- Pennathur A, Qureshi I, Schuchert MJ, et al. Comparison of surgical techniques for early-stage thymoma: feasibility of minimally invasive thymectomy and comparison with open resection. J Thorac Cardiovasc Surg 2011;141:694-701. [Crossref] [PubMed]
- Bachmann K, Burkhardt D, Schreiter I, et al. Long-term outcome and quality of life after open and thoracoscopic thymectomy for myasthenia gravis: analysis of 131 patients. Surg Endosc 2008;22:2470-7. [Crossref] [PubMed]
- Jun Y, Hao L, Demin L, et al. Da Vinci robot-assisted system for thymectomy: experience of 55 patients in China. Int J Med Robot 2014;10:294-9. [Crossref] [PubMed]
- Zhang H, Chen L, Zheng Y, et al. Robot-assisted thymectomy via subxiphoid approach: technical details and early outcomes. J Thorac Dis 2018;10:1677-82. [Crossref] [PubMed]
- Marulli G, Rea F, Melfi F, et al. Robot-aided thoracoscopic thymectomy for early-stage thymoma: a multicenter European study. J Thorac Cardiovasc Surg 2012;144:1125-30. [Crossref] [PubMed]
- Luzzi L, Corzani R, Burali G, et al. First case of combined robot-assisted thymectomy and transaxillary thyroidectomy: technique and robot-docking optimization. J Robot Surg 2017;11:239-41. [Crossref] [PubMed]
- Brown LM, Louie BE. Robot-Assisted Total Thymectomy: How I Teach It. Ann Thorac Surg 2017;103:369-72. [Crossref] [PubMed]
- Hartwich J, Tyagi S, Margaron F, et al. Robot-assisted thoracoscopic thymectomy for treating myasthenia gravis in children. J Laparoendosc Adv Surg Tech A 2012;22:925-9. [Crossref] [PubMed]
- Seong YW, Kang CH, Choi JW, et al. Early clinical outcomes of robot-assisted surgery for anterior mediastinal mass: its superiority over a conventional sternotomy approach evaluated by propensity score matching. Eur J Cardiothorac Surg 2014;45:e68-73; discussion e73.
- Bodner J, Wykypiel H, Greiner A, et al. Early experience with robot-assisted surgery for mediastinal masses. Ann Thorac Surg 2004;78:259-65; discussion 265-6. [Crossref] [PubMed]
- Rückert JC, Swierzy M, Ismail M. Comparison of robotic and nonrobotic thoracoscopic thymectomy: a cohort study. J Thorac Cardiovasc Surg 2011;141:673-7. [Crossref] [PubMed]
- Qian L, Chen X, Huang J, et al. A comparison of three approaches for the treatment of early-stage thymomas: robot-assisted thoracic surgery, video-assisted thoracic surgery, and median sternotomy. J Thorac Dis 2017;9:1997-2005. [Crossref] [PubMed]
- Roviaro GC, Varoli F, Vergani C. Thoracoscopic thymectomy. Semin Laparosc Surg 1997;4:219-22.
- Lin MW, Chang YL, Huang PM, et al. Thymectomy for non-thymomatous myasthenia gravis: a comparison of surgical methods and analysis of prognostic factors. Eur J Cardiothorac Surg 2010;37:7-12. [Crossref] [PubMed]
- Zahid I, Sharif S, Routledge T, et al. Video-assisted thoracoscopic surgery or transsternal thymectomy in the treatment of myasthenia gravis? Interact Cardiovasc Thorac Surg 2011;12:40-6. [Crossref] [PubMed]
- Berman M, Stamler A, Vidne BA, et al. Computer-enhanced thoracoscopic thymectomy with the Zeus telemanipulation surgical system. Interact Cardiovasc Thorac Surg 2003;2:262-4. [Crossref] [PubMed]
- Yim AP. Video-assisted thoracoscopic resection of anterior mediastinal masses. Int Surg 1996;81:350-3. [PubMed]
- Roviaro G, Varoli F, Nucca O, et al. Videothoracoscopic approach to primary mediastinal pathology. Chest 2000;117:1179-83. [Crossref] [PubMed]
- Sugarbaker DJ. Thoracoscopy in the management of anterior mediastinal masses. Ann Thorac Surg 1993;56:653-6. [Crossref] [PubMed]
- Cakar F, Werner P, Augustin F, et al. A comparison of outcomes after robotic open extended thymectomy for myasthenia gravis. Eur J Cardiothorac Surg 2007;31:501-4; discussion 504-5. [Crossref] [PubMed]
- Weksler B, Tavares J, Newhook TE, et al. Robot-assisted thymectomy is superior to transsternal thymectomy. Surg Endosc 2012;26:261-6. [Crossref] [PubMed]
- Balduyck B, Hendriks JM, Lauwers P, et al. Quality of life after anterior mediastinal mass resection: a prospective study comparing open with robotic-assisted thoracoscopic resection. Eur J Cardiothorac Surg 2011;39:543-8. [Crossref] [PubMed]
- O'Sullivan KE, Kreaden US, Hebert AE, et al. A systematic review of robotic versus open and video assisted thoracoscopic surgery (VATS) approaches for thymectomy. Ann Cardiothorac Surg 2019;8:174-93. [Crossref] [PubMed]