
Extended sleeve-lobectomy for centrally located locally advanced non-small cell lung cancer is a feasible approach to avoid pneumonectomy
Introduction
Over the past 20 years, there has been adequate literature showing that bronchoplastic lobectomy offers at least equivalent long-term survival with less postoperative morbidity and mortality and better quality of life than pneumonectomy (PN) (1-4). Thus, bronchoplastic lobectomy is now recommended as the procedure of choice every time it may guarantee a complete resection (5). However, compared to standard sleeve lobectomy (SSL), extended sleeve lobectomy (ESL), defined as an atypical bronchoplasty with resection of more than one lobe, is technically a more demanding procedure, due to greater discrepancy in bronchial calibers, fragility of the distal stump and increased anastomotic site tension. These main technical issues, with consequent concern about an increased risk of bronchial complications and the relatively infrequent occurrence has limited its diffusion with few small retrospective series published, which provide only limited information (6-11). The largest series recently reported in literature showed an overall incidence of anastomotic complications up to 16% with 8% of bronchopleural fistula (BPF) and a very long median hospital stay of 25 days (12). Moreover, even in absence of bronchial complications, due to the small size of the re-implanted bronchus, moderate stricture or angulation at the bronchial anastomosis may be responsible for impaired ventilation of the residual parenchyma, limiting its potential functional benefit.
From an oncological point of view, the main concern is related to the theoretical risk of increased incidence of local recurrence for the less local control of a centrally located non-small-cell lung cancer (NSCLC) compared to that achievable with PN.
The aim of this study was to report our Institutional experience with ESL in 22 patients affected by a centrally located NSCLC, focusing on technical details, post-operative results, recurrence and survival to determine whether ESL can be accepted as a favorable alternative procedure to PN.
We present the following article in accordance with the STROBE Guideline (Available at http://dx.doi.org/10.21037/jtd-20-1241).
Methods
Patient population
Among 884 consecutive patients with primary NSCLC who underwent major lung resection from January 2014 to June 2019, 70 bronchoplastic lobectomies (7.9%) were performed. Twenty-two patients undergoing ESL for centrally located tumors were selected from this series and form the population of this study. The prevalence of ESL among the total number of bronchoplastic lobectomies performed during this period was 31.5%. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and the Harmonized Tripartite Guideline for Good Clinical Practice from the International Conference on Harmonization. Our institutional review board granted approval and waived the requirement for specific informed consent for this retrospective study.
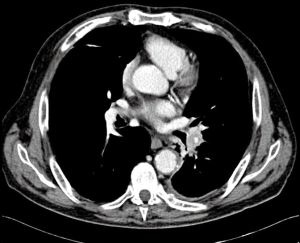
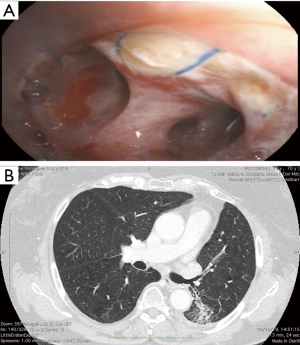
Preoperative assessment included enhanced contrast whole-body computed tomography (CT) scan and 18F-fluorodeoxyglucose whole-body positron emission tomography (PET-CT). A preoperative bronchoscopy was performed in all patients to assess the involvement of the bronchial tree and for diagnostic purpose. In case of suspicious mediastinal lymph nodes at CT or PET-CT-scan, patients underwent cytological or histologic examination by endobronchial or esophageal ultrasound (EBUS/EUS) or video-mediastinoscopy. After a multidisciplinary tumor board discussion, patients with proved N2 disease received neo-adjuvant chemotherapy, unless there was only one station infiltrated, in which case the patient could receive upfront surgery followed by adjuvant chemotherapy. Patients without progression at the CT scan after neo-adjuvant chemotherapy were considered for surgery. Arterial blood gas assessment and spirometry were routinely performed and, in patients with limited lung function, a ventilation/perfusion scan and/or exercise testing was performed. Our strategy was to performed ESL whenever it was technically feasible, even if the patient had sufficient functional pulmonary reserve to tolerate PN, preserving enough healthy lung tissue to fill the thoracic cavity. Traditional indication for ESL was the presence of a large central tumor or hilar lymph nodes infiltrating the main bronchus and extending to another lobe either along the bronchial axis or through the fissure (Figure 1), but not infiltrating far enough to require PN. Basically, PN was performed in case of positive resection margins at the frozen section or in case of macroscopic findings that were inconsistent with the viability of the spared parenchyma or anastomotic site. In addition to the preoperative tests described before, all patients undergoing PN had quantitative lung perfusion scan. Predicted post-operative (ppo) FEV1 and ppo-DLCO had to be more than 35% to accept the patients as candidate for PN. In borderline cases, patients were referred for formal cardiopulmonary exercise testing studies. A maximum oxygen consumption (VO2) peak of <13 mL/kg/min was considered a strong indication that the patient is a high risk for surgery. NSCLC histology was classified according to the most recent WHO classification (13). Tumor, node and metastasis (TNM) stages were assessed postoperatively according to the most recent International Association for the study of Lung Cancer classification (14).
Postoperative complications and mortality, defined as any death within the 30 days following surgery, or during the same hospitalization were analyzed, as well as overall survival (OS) and disease-free survival (DFS). A loco-regional recurrence was defined as any recurrence in the ipsilateral hemithorax such as bronchial stump or anastomosis, ipsilateral mediastinal lymph nodes or structures. Distant recurrence was defined as any recurrence at distant organs or in the contralateral lung. All the patients completed the follow-up and were included in the survival analysis. The last follow-up examination was December 2019.
Operative technique
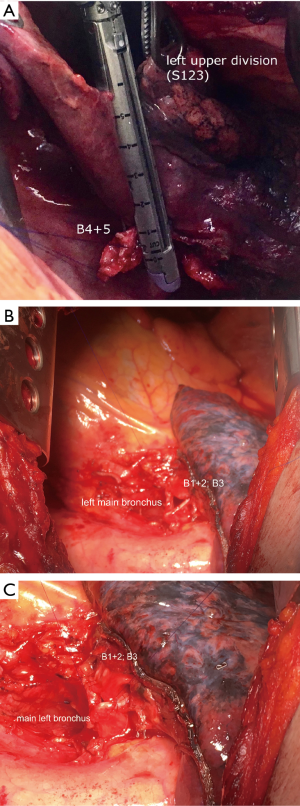
The surgical approach was through a muscle-sparing posterolateral thoracotomy in the fifth intercostal space. Routine systematic mediastinal lymph node dissection was always performed before cutting the bronchus to avoid further dissection around it after completion of the anastomosis. Careful dissection around bronchi was performed avoiding electrocautery to maintain adequate blood supply. Once the feasibility of the bronchoplastic procedure was ascertained, circumferential resection of the main bronchus was associated, when necessary, with pulmonary artery resection to obtain wide and radical oncological margins. When possible, we cut the distal bronchus close to the segmental origin, but sometimes we made the bronchotomy at its branching point. We routinely checked proximal and distal margins with frozen sections and, if the margins were positive, PN was considered. The segment was removed en-bloc with the lobe(s), creating the intersegmental plane with staplers to prevent or minimize air leakage (Figure 2A). We routinely incise the inferior pulmonary ligament to reduce the tension of the bronchial anastomosis; intrapericardial release was also performed in case of ESL types A and B. The bronchial anastomosis was performed with double armed 4/0 polypropylene starting on the deepest site of bronchial stumps and continuing over the anterolateral part in the established single running suture and then using the same single running suture for the rest of posterior part. Bronchial caliber discrepancies were compensated by spacing the suture along the whole circumference of the bronchus. This technique gently stretches and enlarges the circumference of the distal bronchus, with the proximal one working as a stent (Figure 2B,C). Anastomosis coverage was not routinely used, because considered not necessary. Two 30 F chest tubes (on moderate suction, discontinued as soon as possible) were placed to encourage the re-expansion of the residual parenchyma. A routine bronchoscopy was done at the end of operation, before discharge and every time we suspected atelectasis, BPF or in case of persistent air leak.
Statistical analysis
Statistical analysis was performed using SPSS 24.0 software (IBM SPSS Statistics for Machintosh, Version 24.0. Armonk, NY: IBM Corp). Continuous variables are expressed as median and range and compared with Mann-Whitney test. Categorical variables were resumed with percentages and were analyzed using the χ2 test or the Fisher exact test as appropriate. The Kaplan-Meier method was used to calculate overall and DFS. OS was calculated from the date of operation to death or date of the last follow-up (December 2019); DFS was calculated from the date of operation to the date of the first evidence of recurrence. Differences in OS and DFS between ESL and PNs were evaluated by log-rank analysis. Significance was defined as P<0.05.
Results
Preoperative patients’ characteristics are showed in the Table 1. Eight (36.4%) patients were older than 70 years, 10 (45.5%) had modified Charlson co-morbidity index (mCCI) values more than 2 and 14 (63.6%) had an American Society of Anesthesiologists (ASA) score of 3. Six (27.3%) patients had been preoperatively considered unfit for a PN due to predicted postoperative forced expiratory volume in 1 second (FEV1) and/or diffusing capacity of the lung for carbon monoxide (DLCO) of less than 35%. CT4 disease was diagnosed in 3 (13.6%) patients for mediastinal invasion (n=1), nodule in another lobe (n=1) and tumor diameter (n=1). Neo-adjuvant chemotherapy was administered in 7 (31.8%) out of the 10 patients that showed a cN2 disease.
Full table
According to Okada classification, 8 cases of type A ESL (resection of right upper plus middle lobe ± segment 6), one case of type B (resection of left upper lobe + segment 6) and 13 cases of type C (resection of left lower lobe + lingulectomy) ESL were performed. Six patients undergoing type A and one patient undergoing type B ESL had a large central tumor originating in the main bronchus and extending distally, along the bronchial axis or through the fissure up to the other lobe. In two other patients, the decision to perform a type A ESL was made intraoperatively after an attempted standard sleeve lobectomy because of positive bronchial resection margins. The main indication for type C ESL instead, was related to a tumor arising at the origin of the left lower lobe bronchus and infiltrating the lower border of the lingular bronchus or the pulmonary artery branches for the lingula directly (n=6) or through the involvement of station 11L interlobar lymph nodes (n=7). Altogether, the feasibility of ESL was predicted preoperatively on the basis of examinations in 16 (72.6%) patients and was considered during surgery in the 6 (27.3%) other patients. Concomitant pulmonary angioplasty was done in 7 (31.8%) patients, including a pulmonary arterioplasty (all arterial sleeve resections) in 6 patients and pulmonary venoplasty in 1. The pulmonary arterioplasty was frequent in type A and B (6 out of 9, 66.6%) and never necessary in type C. Complete resection was achieved in all cases. Squamous cell carcinoma was the most common histologic type (n=14, 63.6%). All 11 (50%) patients with pN2 disease received adjuvant treatments (Table 2). There was no postoperative mortality, but 54.5% (n=12) of the patient developed at least one postoperative complication. Major postoperative complications developed in 2 (9.1%) patients. One patient who underwent ESL type C developed an anastomotic dehiscence and empyema that healed with a small thoracostomy in few weeks. Another patient developed pulmonary embolism. The most common complication was sputum retention requiring bronchoscopy (n=6; 27.3%), that lead to pneumonia of residual segments in 2 patients (9.1%). We also observed atrial fibrillation in 4 patients (18.2%) and prolonged air leak in 2 other patients (9.1%). Median length of stay was 10 days (6–44 days). Complete long-term patency of the reconstructed airway was documented in all patients by fiber-optic bronchoscopy and currently no patient has developed bronchial stenosis (Figure 3).
Full table
Spirometry was performed 3 months after surgery on the most recent 9 consecutive patients. Mean postoperative decrease in FEV1 was 246 mL equivalent to a loss of 10% of the preoperative volumes.
During follow-up, one patient successfully underwent to a wedge resection of the opposite lung with the excision of a second primary NSCLC.
At the median follow-up of 21 months (4–57 months), the recurrence rate was 54.5%. Two (9.1%) patients had a loco-regional recurrence (1 in the mediastinal lymph nodes, 1 in the re-implanted lung), 8 (36.4%) had distant metastasis only and 2 (9.1%) both (mediastinal lymph nodes, liver and kidney). No endobronchial or perianastomotic recurrence occurred. The 3-year OS rate was 45% with a median survival of 33 months (95% CI: 17.7–48.2 months). The median DFS was 28 months (95% CI: 5.7–50.3 months).
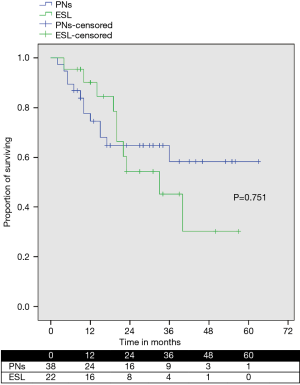
During the same period, we also performed 38 PNs, including 4 in which an ESL was unsuccessfully attempted for microscopic positive bronchial margins at the frozen section (2 cases) or to extensive involvement of the other lobe (2 cases). OS was similar between patients who underwent to ESL and PN (Figure 4).
Discussion
SSL has been proven to be a safe and valid alternative to PN in case of centrally located NSCLC (1-4). When the tumor infiltrates more than one lobe, precluding a radical resection with a SSL, it is still possible to avoid PN performing an ESL. However, this procedure is more technically demanding and concern of an increased risk of bronchial complications has limited its diffusion, with consequent inconclusive and scattered data in literature (6-12). Okada et al. were the first to report an important series in 1999, with 15 (9.6%) ESLs out of 157 bronchoplastic lobectomies (6). Chida et al. reported 23 (42%) ESLs out 55 bronchoplastic lobectomy (10) and Berthet et al. 27 (26%) out of 101 bronchoplastic lobectomies (8). The largest series, from Korea, reported 63 (11.2%) ESLs out of 540 SSLs in about 21 years. We performed 22 (31.5%) ESLs out of 70 bronchoplastic lobectomies in about 5 years. From the technical point of view the main issues of ESL are: (I) the discrepancy of bronchial stumps, with (II) the fragility of the distal (segmental) one, (III) the potentially increased anastomotic tension and (IV) the small amount of spared pulmonary parenchyma to fill the whole chest cavity. ESLs requires an anastomosis between the proximal horseshoe-shape bronchial stump and distal segmental bronchus which has scattered cartilage with significant size discrepancy that in our series was always compensated by a radial distribution of the running suture, spacing the stitches along the whole circumference of the bronchus, without recurring to other technique like plication of the membranous part of the proximal bronchus (6-8), oblique section of the distal one (12,15,16) or telescoping bronchial anastomosis (10). The continuous suture is faster than the interrupted one and can be completed with a single thread reducing the amount of foreign material. It is also easily reproduced when the bronchial sleeve resection is performed with a minimally invasive approach (17). The successful use of a single running suture with a non-absorbable monofilament thread in tracheobronchial sleeve resection has been previously published by the Brompton group in 1999 (18) and its advantages has been demonstrated during lung transplantation by the Vienna lung transplant group (19). In our experience anastomotic bronchial complications occurred in only one case (4.5%) of ESL type C, in which a BPF developed in the seventh postoperative day. The complication was due to the increased anastomotic tension and to the large size discrepancy between the left main and the superior segment bronchus. Although with the running suture there was a fear of complete disruption of the anastomosis, this healed within a few weeks thank to a small thoracostomy. In the whole series, no case of bronchial stenosis was observed and long-term patency of bronchial anastomosis was obtained in all cases. These results compare favorably with those of other series of ESLs in which the bronchial complications ranged from 5% to 16% (6-12).
A sleeve resection and reconstruction of the pulmonary artery was necessary in 6 out of 9 cases of type A or B ESL (never in case of type C), while in other series many types of arterial reconstruction have been used, including the insertion of a pericardial conduit (8,16). We believe that the long resection of the bronchial tree in the type A and B often requires a short arterial resection to avoid its kinking. The major difference in the bronchial reconstruction between type A or B and type C is the angle of the proximal and distal bronchus that in type C is at the 90° respect to the proximal one (20). This makes the realignment of the two stumps more difficult, and the reorientation of the superior segment can sometimes lead to stretch the pulmonary vein with consequent impaired venous flow and eventually thrombosis (12,16). To prevent this dreadful complication, it can be useful to keep the proximal bronchial stump 2–3 mm longer, if oncologically feasible, paying a tribute in terms of reduced blood supply, but resulting in a tension-free anastomosis without squeezing the vein. Thrombosis of the pulmonary vein has been reported even after type A or B ESL as a result of overstretching of the inferior pulmonary vein (6). We did not observe this complication, probably because in all cases the pulmonary ligament was divided and a U-shape pericardial incision was added for type A and B ESLs, as suggested by many Authors (6,8,12,16). Completion PN was reported in many series ranging from 5.5% to 11% for BPF or pulmonary vein thrombosis (6,12,15-17). Thus, this possibility should be kept in mind, since a delay in treatment could be fatal, especially in case of pulmonary vein thrombosis.
Although the risk of bronchial and vascular complications could be high, the absence of operative mortality in our study as well as in many other reports so far (6-11) and the low postoperative mortality (3%) reported in the largest series (12), corroborate the use of ESL to replace PN, when oncologically adequate. We considered these complex bronchoplastic procedures safe even after neo-adjuvant chemotherapy, although the number of patients who received induction chemotherapy in our study is clearly too small (7 patients) to draw definitive conclusions. Sputum retention was the most common complication observed (n=6, 27.3%), leading to pneumonia of the residual segments in two cases (6-12). Therefore, repeated bronchoscopies, aggressive physiotherapy, early mobilization and antibiotic therapy are mandatory to prevent subsequent life-threatening complications. In these cases, we consider useful the administration of low doses of steroids, as suggested by the group of Rome (21) for the anti-oedema effect particularly useful when the bronchial reconstruction involves small caliber bronchi.
Another concern for performing an ESL is the potentially increased incidence of local recurrence compared to PN, because of closer margins obtained in centrally located tumors. However, the local control of ESL does not seem inferior to that achieved after SSL or PN. In fact, local recurrence rate after ESL is reported to be very rare in recent publications, ranging from 0 to 8% (6-12). Compared to these reports, we observed a higher incidence of local recurrence (18.2%). However, we consider this result acceptable, considering that our series includes patients with locally advanced disease, of which 11 (50%) had pathological N2 disease. The oncological adequacy of the surgical procedure is supported also by the absence of local recurrence at the level of bronchial anastomosis; furthermore, there was no difference in midterm survival between patients receiving an ESL and those undergoing a PN during the same period (P=0.75). Many patients in our series were harboring N1 or/and N2 disease, where the indication to surgery and especially to bronchoplastic procedures has to be carefully evaluated. Some Authors have reported that 5-year survival in patients with N2 disease treated by sleeve lobectomy is close to 0 (21,22). In our experience, although limited by a short follow-up and a small number of patients, the 42% 3-year OS in patients with N2 disease (data not shown) is comparable to the results of conventional lung surgery for NSCLC and not inferior to other series of patients treated with radiation-therapy and chemo-therapy without surgery (23). So, we consider ESL an adequate procedure, not only in patients with N0 disease, but also in patients with nodal involvement.
The 10% decrease of the preoperative respiratory function 3 months after ESL suggests that the preserved areas of lung parenchyma significantly contributes to postoperative respiratory function, limiting the important impact of pulmonary volume loss after PN with potential positive implications. Some patients considered unfit for PN, can still be operated on with benefit. Preservation of lung parenchyma also permits for patients who will develop a second primary lung cancer to be consider for surgery again, as one case in our experience. Finally, patients who need adjuvant chemotherapy can tolerate this complex regimen much better after ESL than after PN. In our series all eleven patients with pN2 disease received and complete the planned adjuvant therapy.
The main limitations of this study are its retrospective nature, the small sample size and the short follow-up, due to the recent time of starting this activity. However, the recent period analyzed ensures a homogeneous population, treated by the same surgical group, with the same indications, surgical technique and pattern of care, in absence of historical bias.
Conclusions
ESL is a safe and effective procedure that should be used not only in patients with limited pulmonary reserve but also it could be recommended as an alternative procedure whenever it may guarantee a complete resection. An aggressive perioperative monitoring and management is the key to prevent and successfully treat the post-operative complications which, although quite common, are very rarely life threatening. In our experience, the single running suture provides excellent results even in case of relevant bronchial mismatch.
Acknowledgments
Presented at European Association for Cardio-Thoracic Surgery (EACTS) XXXIII Annual Meeting, Lisbon 3-5th October 2019.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/jtd-20-1241
Data Sharing Statement: Available at http://dx.doi.org/10.21037/jtd-20-1241
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd-20-1241). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Our institutional review board granted approval and waived the requirement for specific informed consent for this retrospective study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Rea F, Marulli G, Schiavon M, et al. A quarter of a century experience with sleeve lobectomy for non-small cell lung cancer. Eur J Cardiothorac Surg 2008;34:488-92. [Crossref] [PubMed]
- Rendina EA, Venuta F, De Giacomo T, et al. Parenchymal sparing operations for bronchogenic carcinoma. Surg Clin North Am 2002;82:589-609. [Crossref] [PubMed]
- Park JS, Yang HC, Kim HK, et al. Sleeve lobectomy as an alternative procedure to pneumonectomy for non-small cell lung cancer. J Thorac Oncol 2010;5:517-20. [Crossref] [PubMed]
- Pagès PB, Mordan P, Renaud S, et al. Sleeve lobectomy may provide better outcomes than pneumonectomy for non–small cell lung cancer. A decade in a nationwide study. J Thorac Cardiovasc Surg 2017;153:184-95.e3. [Crossref] [PubMed]
- Detterbeck FC, Lewis SZ, Diekemper R, et al. Executive summary: diagnosis and management of lung cancer: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:7S-37S. [Crossref] [PubMed]
- Okada M, Tsubota N, Yoshimura M, et al. Extended sleeve lobectomy for lung cancer: the avoidance of pneumonectomy. J Thorac Cardiovasc Surg 1999;118:710-3; discussion 713-4. [Crossref] [PubMed]
- Yamamoto K, Miyamoto Y, Ohsumi A. Sleeve lung resection for lung cancer: analysis according to the type of procedure. J Thorac Cardiovasc Surg 2008;136:1349-56. [Crossref] [PubMed]
- Berthet JP, Paradela M, Jimenez MJ, et al. Extended sleeve lobectomy: one more step toward avoiding pneumonectomy in centrally located lung cancer. Ann Thorac Surg 2013;96:1988-97. [Crossref] [PubMed]
- Toyooka S, Soh J, Yamamoto H, et al. Extended sleeve lobectomy after induction chemoradiotherapy for non-small cell lung cancer. Surg Today 2015;45:1121-6. [Crossref] [PubMed]
- Chida M, Minowa M, Miyoshi S, et al. Extended sleeve lobectomy for locally advanced lung cancer. Ann Thorac Surg 2009;87:900-5. [Crossref] [PubMed]
- Hishida T, Aokage K, Yoshida J, et al. Extended bronchoplasty for locally advanced left lower lobe lung cancer: surgical technique and outcomes. Interact Cardiovasc Thorac Surg 2018;27:602-5. [Crossref] [PubMed]
- Hong TH, Cho JH, Shin S, et al. Extended sleeve lobectomy for centrally located non-small-cell lung cancer: a 20-year single-centre experience. Eur J Cardiothorac Surg 2018;54:142-8. [Crossref] [PubMed]
- Travis WD, Brambilla E, Muller-Hermelink HK, et al. Tumors of Lung. In: Pathology and genetics of a tumors of the lung, pleura, thymus and heart. Lyon: IARC; 2004:13.
- Goldstraw P, Crowley J, Chansky K, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol 2007;2:706-14. [Crossref] [PubMed]
- Miyoshi S, Tamura M, Araki O, et al. Telescoping bronchial anastomosis for extended sleeve lobectomy. J Thorac Cardiovasc Surg 2006;132:978-80. [Crossref] [PubMed]
- Suzuki K. Extended Sleeve Resection for Lung Cancer. Thorac Surg Clin 2018;28:291-7. [Crossref] [PubMed]
- Pan X, Chen Y, Shi J, et al. Robotic assisted extended sleeve lobectomy after neoadjuvant chemotherapy. Ann Thorac Surg 2015;100:e129-31. [Crossref] [PubMed]
- Kutlu CA, Goldstraw P. Tracheobronchial sleeve resection with the use of a continuous anastomosis: results of one hundred consecutive cases. J Thorac Cardiovasc Surg 1999;117:1112-7. [Crossref] [PubMed]
- Aigner C, Jaksch P, Seebacher G, et al. Single running suture – the new standard technique for bronchial anastomoses in lung transplantation. Eur J Cardiothorac Surg 2003;23:488-93. [Crossref] [PubMed]
- Tsubochi H, Kanai Y, Tezuka K, et al. Extended sleeve lobectomy for interlobar lymph node metastasis invading the bronchus from peripheral lung cancer. Gen Thorac Cardiovasc Surg 2011;59:515-7. [Crossref] [PubMed]
- Maurizi G, Ciccone AM, Vanni C, et al. Reimplantation of the upper lobe bronchus after lower sleeve lobectomy or bilobectomy: long-term results. Eur J Cardiothorac Surg 2018;53:1180-5. [Crossref] [PubMed]
- Yildizeli B, Fadel E, Mussot S, et al. Morbidity, mortality, and long-term survival after sleeve lobectomy for non-small cell lung cancer. Eur J Cardiothorac Surg 2007;31:95-102. [Crossref] [PubMed]
- Albain KS, Swann RS, Rusch VW, et al. Radiotherapy plus chemotherapy with or without surgical resection for stage III non-small-cell lung cancer: a phase III randomised controlled trial. Lancet 2009;374:379-86. [Crossref] [PubMed]


