
Pulmonary enteric adenocarcinoma with villin brush border immunoreactivity: a case report and literature review
Introduction
Pulmonary enteric adenocarcinoma (PEAC) is a rare primary lung adenocarcinoma with its histological morphology similar to that of metastatic colorectal carcinoma (MCC). It was originally described in 2005 by Inamura et al. based on a series of 7 cases (1), and until now, there are only 16 cases reported. Due to rare documents, it has not yet been listed in the recent World Health Organization (WHO) classification of lung tumors. International multidisciplinary classification of lung adenocarcinoma was published in early 2011 by International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society (2). In this document, PEAC was classified as a rare variant of invasive adenocarcinoma which was parallel to invasive mucinous adenocarcinoma (formerly mucinous bronchioloalveolar carcinoma), colloid adenocarcinoma and fetal adenocarcinoma.
Herein, we describe the clinicopathologic features of 1 case of PEAC with morphology similar to that of MCC and expression of Villin in the brush border of tumor cells.
Case report
A 61-year-old female nonsmoker presented with a chief complaint of fever and cough. Chest computed tomography (CT) scan showed partial consolidation of the right middle lobe and patchy shadow in the right lower lobe. Fever and cough were not completely relieved after two weeks of anti-inflammatory treatment. A second CT revealed a lump (6 cm × 5 cm ×3 cm) in the right middle lobe (Figure 1). A subsequent right middle lobectomy was performed. In the resected lobe, we found a lump with the dimension of 5 cm × 4 cm × 3 cm. Cut section of the lump was grey and scattered with necrosis. Pleura was locally involved.
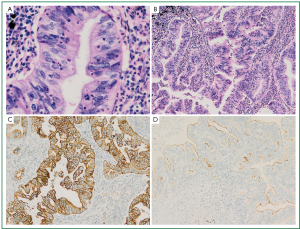
Histological examination of the tumor showed a typical nestlike structure and irregular glandular cavity with central necrosis, which were very similar to that of MCC. Neoplastic cells were tall-columnar with eosinophilic cytoplasm, round or ovoid nuclei, prominent nucleoli and mitotic figure (Figure 2A). In some areas tumor cells exhibited an irregular cribriform pattern. In the periphery of the tumor, we observed non-classical lepidic growth pattern as seen in cases with mucinous adenocarcinoma. The mesenchyma was composed of loose fibrous tissue and inflammatory cells like lymphocyte and neutrocyte, which were around the serrated glands (Figure 2B). Immunohistochemical analysis showed the tumor cells were diffusely positive for cytokeratin (CK) 7 (Figure 2C), partially positive for CK20, strongly positive for villin in the brush border (Figure 2D), but negative for TTF-1.
Discussion
PEAC is a rare type of lung adenocarcinoma which has been reported in 16 cases to date (1,3-6). A case of primary pulmonary tumor with enteric differentiation was reported by Tsao et al. (7) in 1991. Although it was named pulmonary adenocarcinoma with enteric differentiation, but its histological features were not consistent with that of PEAC. Recent descriptions of PEAC tend to have semblable histological morphology. However, PEAC had different names in the previous literatures such as pulmonary adenocarcinoma with intestinal differentiation, pulmonary intestinal-type adenocarcinoma and pulmonary adenocarcinoma with enteric differentiation. Because the histological heterogeneity of pulmonary carcinoma is well recognized, PEAC was defined as primary pulmonary adenocarcinoma with a predominant component (>50%) of intestinal differentiation with tumor cells positive for at least one intestinal markers (such as CDX-2, CK20, MUC2) (2). If tumor cells were negative for any intestinal marker, the tumor should be termed lung adenocarcinomas with enteric morphology (2).
Previous reports suggest both sexes have a similar incidence of PEAC, with a median age of 66 (range, 51-82) and a mean diameter of 3.5 cm. Of the 16 reported cases, 11 tumors were in the right lung. Most patients are smokers (14/16), suggesting smoking being likely an causative factor for PEAC. Initial symptoms can be cough, fever, hemoptysis and chest pain, whereas some cases were not confirmed until physical examination, with no significant symptoms being perceived in daily activities.
Morphologically, PEAC is composed of moderate-to-well differentiated glands as seen in colorectal carcinoma. In some areas tumor cells grow in an irregular cribriform pattern. Another significant feature is dirty necrosis in the lumen which can also be observed in colorectal carcinoma. This necrosis pattern was detectable in nearly all reported cases. Tumor cells are tall columnar with brush border, eosinophilic cytoplasm, ovoid nuclei and occasional prominent nucleoli. Nuclei arrange in a palisading pattern and the polarity of nuclei is well preserved. But in areas with poor differentiation, the polarity is partially lost. The mesenchyma shows inflammatory changes, infiltrated with inflammatory cells like lymphocyte and neutrocyte. Actually, all the aboving histological morphology can be encountered in MCC, therefore it is critical to distinguish between PEAC and MCC. The histologic heterogeneity of PEAC is so marked that in all the cases Inamura had reported, other components, like lepidic growth and clear cell cytoplasmic changes, are present besides intestinal differentiation (1). These heterogeneous components give a clue for the diagnosis of PEAC. In this case we observed non-classical lepidic growth pattern as seen in some mucinous carcinoma. Therefore paraffin blocks originating from a sample should be made as many as possible, in the hope of finding components other than enteric adenocarcinoma. The diagnosis of PEAC should not be made by small biopsies (bronchoscopic, needle, or core biopsies). However, one case of PEAC was reported in which tumor is exclusively composed of components resembling colorectal carcinoma (5). In such cases, it is difficult to differentiate it from MCC by histology alone. Hence it is important to search for primary lesion in the intestines and make it clear whether the patient suffers from intestinal carcinoma. Positron emission tomography (PET-CT) may be useful in detecting small focus in intestinal carcinoma (8). In the present case, the patient underwent PET scan which showed no mass in other organs.
Immunohistochemical analysis provides assistance for the differentiation between PEAC and MCC (Table 1). Previous reports showed CK7 positive and CK20 negative in the most cases of PEAC, and the positive rates for TTF-1 and CDX-2 were 58.8% and 43.8%, respectively. A total of 24 cases of MCC in lungs reported by Inamura (1) and Yousem (3) exhibited the same imunophenotypic profiles (CK7-, CK20+, CDX-2+, TTF-1–) as that of the primary colorectal adenocarcinoma, except 2 cases with CK20 negative. In the differentiation between PEAC and MCC, CK7 is relatively specific and sensitive, whereas TTF-1 is specific but not so sensitive. CK20 and CDX-2 are of some significance with poor specificity. There were two cases of PEACs with immunohistochemical findings indistinguishable from colorectal carcinoma (5,6). On these occasions, it is imperative to distinguish it from MCC. For the differential diagnosis, efforts should be made to search for the primary lesion and other components than intestinal carcinoma. Interestingly, villin expression follows an intestinal pattern in the present case. In other words, villin is expressed in the brush border. It is known that villin is not expressed diffusely in the brush border in primary pulmonary adenocarcinoma except for a few cases of bronchioalveolar carcinoma (9,10). But in cases of MCC, villin is expressed in the brush border (9,11). Hence we can’t distinguish PEAC from MCC just on the basis of villin expression pattern in the brush border (10,11). However, villin can be used as a marker to determine the presence of enteric differentiation in cases of PEAC.
Table 1
| Sex/Age(y) | Size(cm)/Site | Smoking | Immunohistological results
|
Follow-up (mo) | Reference | |||
|---|---|---|---|---|---|---|---|---|
| CK7 | CK20 | TTF-1 | CDX-2 | |||||
| M/NA | 5.0/RUL | Yes | + | – | + | – | 60/D | (1) |
| M/NA | 4.0/LUL | Yes | + | – | + | + | 47/D | (1) |
| F/NA | 2.6/LLL | Yes | + | P+ | – | P+ | 43/A | (1) |
| M/NA | 3.4/RLL | Yes | + | P+ | – | P+ | 43/A | (1) |
| M/NA | 2.3/RLL | Yes | + | + | – | + | 30/A | (1) |
| M/NA | 1.7/RLL | Yes | + | – | P+ | P+ | 16/A | (1) |
| M/NA | 3.9/LUL | Yes | + | – | – | – | 12/A | (1) |
| F/74 | 3.6/RUL | Yes | + | – | + | – | 26/D | (3) |
| F/70 | 1.7/RUL | Yes | + | – | + | – | 18/D | (3) |
| M/82 | 6.5/RUL | Yes | + | – | P+ | – | 5/D | (3) |
| F/63 | 1.5/RUL | Yes | + | – | + | – | 7/A | (3) |
| F/73 | 7.0/LLL | Yes | + | – | + | – | 3/A | (3) |
| F/57 | 2.0/RUL | Yes | + | – | + | – | 2/A | (3) |
| M/69 | 2.5/RLL | NA | + | – | + | NA | NA | (4) |
| F/51 | 3.3/LLL | Yes | – | + | – | + | 10/A | (5) |
| F/51 | 3.0/LLL and 1.0/RUL | No | – | + | – | + | 48/A | (6) |
| F/61 | 5.0/RML | No | + | P+ | – | – | 6/A | Present case |
A, alive; CK, cytokeratin; D, died; LLL, left lower lobe; LUL, left upper lobe; NA, not available; P, partially; RLL, right lower lobe; RUL, right upper lobe; +, positive; –, negative.
The present case was followed up for 6 months with no recurrence or metastasis. Of the other 15 cases with follow-up, 5 died, 2 of which died of tumor itself definitely. There was a survived patient showing locally recurrent mass.
To be concluded, we reported a case of PEAC with positive expression of villin in the lumen side of the gland. PEAC is a rare lung tumor which should be distinguished from MCC according to medical history, imaging appearance, histological and immunohistochemical features. Rare documentation and short period of follow-up lead to little understanding of this tumor. More cases are needed to further explore this tumor.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Inamura K, Satoh Y, Okumura S, et al. Pulmonary adenocarcinomas with enteric differentiation: histologic and immunohistochemical characteristics compared with metastatic colorectal cancers and usual pulmonary adenocarcinomas. Am J Surg Pathol 2005;29:660-5. [PubMed]
- Travis WD, Brambilla E, Noguchi M, et al. International association for the study of lung cancer/american thoracic society/european respiratory society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol 2011;6:244-85. [PubMed]
- Yousem SA. Pulmonary intestinal-type adenocarcinoma does not show enteric differentiation by immunohistochemical study. Mod Pathol 2005;18:816-21. [PubMed]
- Maeda R, Isowa N, Onuma H, et al. Pulmonary intestinal-type adenocarcinoma. Interact Cardiovasc Thorac Surg 2008;7:349-51. [PubMed]
- Li HC, Schmidt L, Greenson JK, et al. Primary pulmonary adenocarcinoma with intestinal differentiation mimicking MCC: case report and review of literature. Am J Clin Pathol 2009;131:129-33. [PubMed]
- Hatanaka K, Tsuta K, Watanabe K, et al. Primary pulmonary adenocarcinoma with enteric differentiation resembling MCC: a report of the second case negative for cytokeratin 7. Pathol Res Pract 2011;207:188-91. [PubMed]
- Tsao MS, Fraser RS. Primary pulmonary adenocarcinoma with enteric differentiation. Cancer 1991;68:1754-7. [PubMed]
- Abdel-Nabi H, Doerr RJ, Lamonica DM, et al. Staging of primary colorectal carcinomas with fluorine-18 fluorodeoxyglucose whole-body PET: correlation with histopathologic and CT findings. Radiology 1998;206:755-60. [PubMed]
- Tan J, Sidhu G, Greco MA, et al. Villin, cytokeratin 7, and cytokeratin 20 expression in pulmonary adenocarcinoma with ultrastructural evidence of microvilli with rootlets. Hum Pathol 1998;29:390-6. [PubMed]
- Nambu Y, Iannettoni MD, Orringer MB, et al. Unique expression patterns and alterations in the intestinal protein villin in primary and metastatic pulmonary adenocarcinomas. Mol Carcinog. 1998;23:234-42. [PubMed]
- Moll R, Robine S, Dudouet B, et al. Villin: A cytoskeletal protein and a differentiation expressed in some human adenocarcinomas. Virchows Arch B Cell Pathol Incl Mol Pathol 1987;54:155-69. [PubMed]



