Effects of transdermal tulobuterol on dyspnea and respiratory function during exercise in patients with chronic obstructive pulmonary disease
Introduction
Chronic obstructive pulmonary disease (COPD) is a highly prevalent disorder worldwide (1,2). In Japan, it was recently estimated that COPD affects 8.6% of the population (1), increasing to 22% in people with a history of smoking or respiratory symptoms. However, many individuals have undiagnosed COPD, despite routinely visiting medical clinics for the treatment of other diseases (1).
COPD is associated with high mortality rates in many countries, although recent improvements in treatment have tended to reduce COPD-related mortality rates (3). The Global Initiative for Chronic Obstructive Lung Disease (GOLD) (4) and the Japanese Respiratory Society (5) recommend improving and maintaining the improvements in exercise tolerability and physical activity as the main components of COPD management. However, lifestyle interventions are often inadequate, and many patients require pharmacotherapy. Accordingly, β adrenergic agonists are also recommended as part of the treatment of COPD, and should be considered early in the treatment program.
Several clinical trials conducted in Japan (6,7) and in other countries (8-10) have shown that inhaled salmeterol, a long-acting β2 adrenergic agonist, improves respiratory function and exercise tolerability in patients with COPD. In Japan, a transdermal long-acting β2 agonist (transdermal tulobuterol) is also available. In a study of elderly Japanese COPD patients (11), it was reported that adherence to transdermal tulobuterol was significantly better than that to inhaled salmeterol (90.3%±1.6% vs. 75.5%±2.9%). In that study, the 6-min walk distance and quality of life [determined using the St. George’s Respiratory Questionnaire (SGRQ)] improved significantly in the transdermal tulobuterol group but not in the salmeterol group. In an open-label study of Japanese patients with stable COPD, transdermal tulobuterol was as effective as inhaled salmeterol in terms of improvements in spirometric parameters, and the improvement in SGRQ score was greater in the transdermal tulobuterol group than in the salmeterol group (12).
Despite these findings, no studies have reported the effects of transdermal tulobuterol on exercise tolerability. It is important to evaluate these effects because inadequate ventilatory responses and the onset of dyspnea are significant barriers to exercise, and may limit the willingness of patients with COPD to perform regular exercise or physical activities. Therefore, the aim of this study was to examine the effects of transdermal tulobuterol on exercise tolerability, especially dyspnea, in Japanese COPD patients.
Methods
Patients
Japanese males with moderate to severe COPD and who showed good adherence to their COPD therapy were invited to participate in this study. Fifteen patients were initially enrolled, but two were subsequently excluded because they did not have dyspnea. The diagnosis of COPD was retrieved from the patient’s medical records, and was based on GOLD criteria (4) in terms of medical history, radiologic (including high-resolution computed tomography of the chest) and pulmonary (including spirometry) criteria, and the presence of persistent exertional dyspnea with signs of an overdistended lung volume. Moderate to severe COPD was defined as post-bronchodilator forced expiratory volume in 1 s (FEV1)/forced vital capacity (FVC) ratio of <70% and percent predicted FEV1 (%FEV1) <80%. Only patients whose condition had remained stable for ≥1 month were eligible. Patients with any of the following were excluded: respiratory tract infection within the preceding 4 weeks, bronchial asthma, hypertension, heart disease, thyroid disease, diabetes, or skin disease. Patients contraindicated to β2 agonists or who required corticosteroids were also excluded from the study. All patients gave written informed consent to participate in this study.
Study design
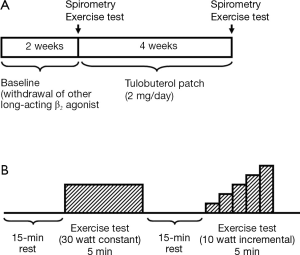
The study was conducted at a respiratory medicine outpatient clinic in Juntendo University Hospital in Japan. Patients who were using another β2 agonist were to enter a 2-week washout period before the baseline assessments. Patients who were using short- or long-acting muscarinic antagonists continued these drugs in the treatment period. No patients were on steroids (oral or inhaled) during the time of the study. After the baseline assessment, patients started treatment with 2 mg transdermal tulobuterol once daily for 4 weeks (Figure 1). Pulmonary function tests and exercise tests were performed at baseline and after 4 weeks of treatment with transdermal tulobuterol. The patients were given standard lifestyle advice consistent with clinical practice in Japan.
The study was approved by the ethical committee of Juntendo University (approval number: 24-056) and was registered on the University Hospital Medical Information Network (identifier UMIN000012795). This study was performed between March 2011 and March 2013.
Pulmonary function test
Pulmonary function tests were performed at baseline and after 4 weeks of treatment with transdermal tulobuterol. The effects of transdermal tulobuterol on spirometry were assessed in terms of FEV1, %FEV1, FEV1/FVC, peak expiratory flow (PEF), and inhaled capacity (IC). For spirometry, the best of three maximal flow volume curves was analyzed. Respiratory function was measured using a multi-purpose spirometer (Autospirometer SYSTEM 21; Minato Medical Science Co., Ltd., Osaka, Japan).
Exercise test
Pulmonary function and exercise tests were performed at baseline and after 4 weeks of treatment with transdermal tulobuterol. We designed a suitable test for use in this study (Figure 1).
The exercise test consisted of two types of exercise (fixed load and incremental load) on a bicycle ergometer (Rehcor; Lode B. V., Groningen, the Netherlands). After a 2-min warm-up at 0 W, the patients cycled for 5 min at 30 W (fixed load test). After resting for 15 min, the patients cycled for a further 5 min starting at 10 W. In this phase of the test, the load was increased by 10 W at 1-min intervals to a maximum load of 50 W (incremental load test). This load used in the incremental load test was based on the load used in a prior study that examined the effects of anticholinergic drugs on dyspnea and gas exchange in COPD patients (13). The loads used in these tests were considered to represent lower levels of daily activity based on the estimated metabolic equivalents of these loads defined in an earlier report (14). The investigators did not provide any verbal encouragement during the tests.
Minute ventilation (VE), oxygen uptake (VO2), respiratory frequency, carbon dioxide output (VCO2), SpO2, heart rate, blood pressure, and Borg scale were assessed during exercise. Blood pressure was measured using a sphygmomanometer and heart rate was measured by feeling the patient’s pulse every minute during the test. Respiratory function was measured using a SensorMedics Vmax229 metabolic cart (CareFusion Corp., San Diego, CA, USA).
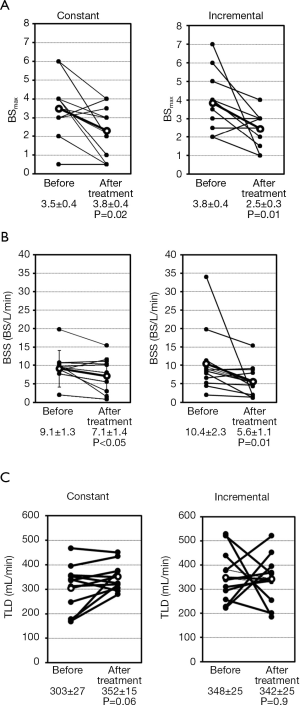
The Borg scale for dyspnea was assessed every minute during the exercise test using a scale ranging from 0 (no breathlessness) to 10 (maximal breathlessness) (15). We asked the patients to point out the grades of modified Borg scale on a panel by a finger during bicycle exercise at every 1 min. Dyspnea was evaluated in terms of the Borg scale slope (BSS) and the threshold load of dyspnea (TLD), as previously described (13), by plotting the patient’s VO2 against the Borg scale, as illustrated in Figure 2. The slope of the regression line was calculated as the BSS. The intercept of the regression line on the x-axis was calculated as the TLD, and corresponds to VO2 at the initial sensation of dyspnea during exercise. The largest Borg scale value reported by each patient was defined as BSmax. Changes in the BSS, TLD, and BSmax were compared before and after treatment to examine the impact of transdermal tulobuterol on dyspnea and exercise tolerance.
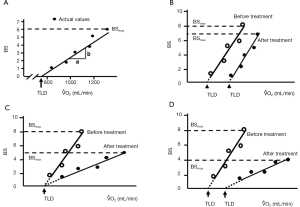
The patterns of changes in the BS-VO2 regression line in patients with decreases in BSmax after treatment with transdermal tulobuterol are shown in Figure 2B-D. In Figure 2B, BSS is unchanged, but TLD increases and BSmax decreases. This indicates that the onset of dyspnea during exercise was reduced, but dyspnea sensitivity was unchanged. Figure 2C shows a patient whose BSS and BSmax decrease, but TLD remains unchanged. This indicates that the onset of dyspnea during exercise was not influenced by transdermal tulobuterol, but dyspnea sensitivity was decreased after treatment. Figure 2D shows a patient whose BSS and BSmax decrease, but TLD increases. This indicates that the onset and sensitivity of dyspnea during exercise were reduced following treatment with transdermal tulobuterol.
The results of the exercise tests were calculated for the constant load and incremental load separately.
Statistical analyses
Date are expressed as means ± standard deviation (SD). Because of the small number of patients enrolled, we assumed that the variables would be non-normally distributed. Therefore, the variables were compared between before and after treatment using Wilcoxon’s matched-pair test for pairwise comparisons. For all analyses, P<0.05 was considered statistically significant. Statistical analyses were conducted using Graphpad PRISM® software version 6.0 (GraphPad Software, San Diego, CA, USA). Sample size and power calculations were not performed.
Results
Patients
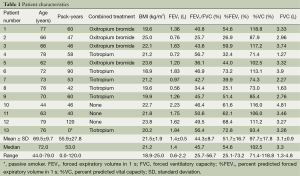
The characteristics of the 13 patients enrolled in the study are summarized in Table 1. The mean ± SD age was 69.5±9.7 years. Twelve were smokers (55.9±27.8 pack-years) and one was a passive smoker. Most of the patients were being treated with a short-acting muscarinic antagonist or a long-acting muscarinic antagonist (tiotropium). Eight patients were using salmeterol before enrollment and entered a washout period before starting transdermal tulobuterol. The patients verbally confirmed that they used the study drug between each visit.
Full table
Effects of transdermal tulobuterol on spirometry
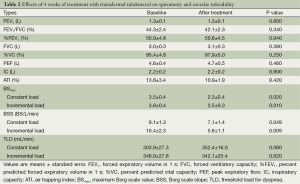
As shown in Table 2, there were no significant changes in any of the spirometric parameters measured at rest between baseline and after 4 weeks of treatment with transdermal tulobuterol.
Full table
Effects of transdermal tulobuterol on exercise tolerability
The changes in BSmax, BSS, and TLD between before and after treatment are shown in Figure 3 for individual patients and the mean values are presented in Table 2. BSmax (Figure 3A, Table 2) decreased significantly from 3.5 to 2.3 in the constant load test (P=0.02) and from 3.8 to 2.5 in the incremental load test (P=0.01). BSS also decreased significantly after treatment with transdermal tulobuterol, from 9.1 to 7.1 BS/L/min in the constant load test and from 10.4 to 5.6 BS/L/min in the incremental load exercise test (P=0.009) (Figure 3B, Table 2). TLD increased slightly, albeit non-significantly, from 303.9 to 352.4 mL/min during the constant load test (P=0.06), but remained unchanged in the incremental load test (Figure 3C, Table 2).
There were no marked changes in SpO2, respiratory frequency, tidal volume, or VE between before and after treatment in the constant load test (data not shown).
Effects of transdermal tulobuterol on blood pressure and heart rate
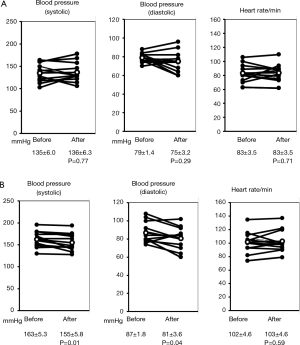
The blood pressures and heart-rates measured at rest before constant load exercise test and at the end of the exercise are shown in Figure 4 for individual patients. Blood pressure (systolic and diastolic) and heart rate at rest showed no significant difference 4 weeks after the administration of transdermal tulobuterol patch. However, both systolic and diastolic pressures at the end of exercise were significantly decreased after treatment with the transdermal tulobuterol from 163±5.3 to 155±5.8 mmHg for systolic and from 87.3±1.8 to 81±3.6 mmHg for diastolic (P<0.05) although the sign was not expected because of the effects of beta agonist. Heart rate had not significantly been changed from 102±4.6 to 103±4.6/min (P=0.59). The same tendency was showed as the incremental load exercise (data not shown).
Discussion
The results of this study showed that 4 weeks of treatment with transdermal tulobuterol significantly reduced subjective assessments of exercise-induced dyspnea (i.e., BSmax and BSS) in constant and incremental load exercise tests in Japanese patients with COPD. Treatment with transdermal tulobuterol also slightly, albeit not significantly, increased TLD during the constant load test but not in the incremental load test. These changes in exercise tolerability occurred in the absence of changes in objective spirometry or SpO2, suggesting that transdermal tulobuterol improved exercise tolerability while maintaining lung function.
β2 agonists and muscarinic antagonists are generally used as the first-choice drugs for COPD because of their abilities to improve obstruction, lung overexpansion, dyspnea, quality of life, and exercise tolerability, and because they may prevent exacerbation of disease. Long-acting β2 agonists may offer some advantages over short-acting β2 agonists in terms of adherence, ease of use, and patient acceptance. Transdermal patches may also improve quality of life and nocturnal symptoms compared with inhaled products.
Although transdermal long-acting β2 agonists (e.g., transdermal tulobuterol) are currently only available in Japan and Korea, several studies have documented their clinical efficacy and acceptability relative to inhaled products (11,12,16,17). For example, in a 12-week open-label study of Japanese patients with stable COPD, Fukuchi et al. (12) reported that transdermal tulobuterol and inhaled salmeterol significantly improved FEV1, FVC, and PEF relative to baseline levels. The SGRQ total score improved significantly in the transdermal tulobuterol group at 8 weeks, but not in the salmeterol group at any time point. Treatment compliance was also significantly greater in the transdermal tulobuterol group (98.5% vs. 94.1%; P<0.05).
Transdermal formulations may overcome some barriers to the use of inhaled products in elderly patients in particular, who may experience difficulty using inhaled devices. For example, in such patients, cognitive disorders may hinder effective treatment, and poor lung function may limit the bioavailability of the drug (11,18). Considering that inhaled salmeterol was also reported to improve respiratory function and exercise tolerability in COPD patients (8-10), it is apparent that there are multiple effective treatment options for COPD, and the choice of drug may depend on the individual patient’s condition and self-care abilities.
Our results differ slightly from those reported by O’Donnell et al. (9) who conducted a study in which 23 patients were treated with salmeterol or placebo in a randomized, double-blind crossover manner. They found that treatment with salmeterol for 2 weeks improved objective respiratory parameters (e.g., IC, tidal volume, and VO2) compared with placebo, and increased peak exercise endurance. However, they observed no change in the subjective BS for dyspnea in either the constant or incremental load exercise tests. There are several possible reasons for the absence of changes in objective spirometry results in the present study, including the short study period, a smaller study population in our study than O’Donnell et al. (9) and the wide variation in responses that potentially masked the changes in spirometry. On the other hand, there is a difference in the constant load exercise protocol between theirs and our study’s; we used a constant load of 30 W, which was maintained for 5 min, whereas a work-rate of 75% of the subject’s maximal work capacity was maintained until the point of symptom limitation in their study. We speculate that a mild exercise load might be more sensitive to disclose symptomatic improvement by a long-acting β2 agonist in COPD.
Although the 6-min walk test is frequently used to evaluate respiratory function in patients with COPD, it is difficult to assess physiological indices (e.g., VO2 and blood pressure) using this method unless walking on a treadmill, which could be disorientating for some patients. Therefore, we used a cycle ergometer method instead, with some similarities to the method used by O’Donnell et al. (9) in terms of the incremental load test.
The exercise protocol used in our study was conducted at a similar level to the 6-min walk test and reflects daily life activities based on the metabolic equivalents reported for the loads used in this study and other physical activities (14). With the exercise test used in our study, we could record the patient’s perceived level of dyspnea and as well as other ventilatory responses to exercise, which may not be possible during the 6-min walk test. Accordingly, the reductions in BSS and BSmax observed in this study are clinically relevant in terms of reducing the likelihood of subjective dyspnea during daily life activities, and that light exercise can be achieved at home during treatment with transdermal tulobuterol, which may improve the prognosis of patients.
Some limitations of this study warrant mentioning, including the small sample size, continuation of another drug during the study, the lack of an objective measure of quality of life, and the fact that it was conducted at a single center. The exercise protocol is dedicated to evaluate dyspnea during exercise and thus may not be a standardized one in clinical practice. In addition, because this study lacked a control group and was conducted in an open-label manner, we cannot exclude the possibility of a placebo or trial-related effect on the changes in subjective parameters, especially BSmax and BSS. It is possible that the improvements in these parameters are related to the patient’s desire to please the investigators by overestimating the improvements in symptoms since it could be hard to set up the comparing placebo control of tulobuterol patch. However, this is unlikely, as these subjective assessments were conducted during a fairly demanding series of tests that were expected to cause discomfort. We did not perform a sample size calculation, so some comparisons may be underpowered. Nevertheless, the results for the main outcomes were statistically significant despite the small number of patients enrolled in this study.
Gagnon et al. (19) reviewed the pathogenesis of hyperinflation in COPD. This showed that reduced lung elastic recoil combined with expiratory flow limitation leads to lung hyperinflation during the course of the disease. Hyperinflation is clinically a key factor of dyspnea, exercise intolerance, skeletal muscle limitations, morbidity, and reduced physical activities levels associated with the disease. In fact, although measurement of expiratory flows is a prerequisite for the diagnosis and staging of COPD, the effects of the disease on static and dynamic lung volumes correlate better with patient symptoms and impairment in functional capacity than spirometric indices of the disease. Moreover, dynamic hyperinflation can occur independently of static hyperinflation. Guenette et al. (20) evaluated the effects of dynamic hyperinflation on dyspnea by comparing a group of well characterized COPD patients who did not acutely increase their end-expiratory lung volume (EELV) during the exercise (nonhyperinflators) with those that did increase their EELV (hyperinflators). The authors were not able to show that the hyperinflators experienced more dyspnea than the nonhyperinflators. Therefore, when these reports were considered to reference, in the present study which pulmonary function indices had not necessarily show an improvement intentionally after treatment with tulobuterol patch in COPD patients, these tulobuterol patch may improve the dyspnea (Borg scale) and quality of life for COPD patients.
Conclusions
In conclusion, the results of the present study indicate that treatment with transdermal tulobuterol for 4 weeks improved self-assessed dyspnea, which was evaluated using a modified Borg scale, in Japanese COPD patients during constant and incremental exercise tests compared with baseline levels. This improvement in dyspnea may encourage patients to perform daily life activities or regular physical activity, which may improve their prognosis. Larger studies with an appropriate control group are warranted to confirm the present findings.
Acknowledgements
Funding: This study was in part supported by a High Technology Research Center Grant from the Ministry of Education, Culture, Sports, Science and Technology, Japan.
Disclosure: The authors declare no conflict of interest.
References
- Minakata Y, Ichinose M. Epidemiology of COPD in Japan. Nihon Rinsho 2011;69:1721-6. [PubMed]
- Rycroft CE, Heyes A, Lanza L, et al. Epidemiology of chronic obstructive pulmonary disease: a literature review. Int J Chron Obstruct Pulmon Dis 2012;7:457-94. [PubMed]
- Horita N, Miyazawa N, Morita S, et al. Long-acting beta-agonists reduce mortality of patients with severe and very severe chronic obstructive pulmonary disease: a propensity score matching study. Respir Res 2013;14:62. [PubMed]
- Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global Strategy for Diagnosis, Management, and Prevention of COPD, 2013. Available online: www.goldcopd.org/
- Japanese Respiratory Society. eds. Guidelines for the diagnosis and treatment of COPD (chronic obstructive pulmonary disease) 2nd Edition, 2004. Tokyo: Medical Review Co., Ltd, 2004.
- Fujimoto K, Kitaguchi Y, Kanda S, et al. Comparison of efficacy of long-acting bronchodilators in emphysema dominant and emphysema nondominant chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis 2011;6:219-27. [PubMed]
- Kurashima K, Hara K, Yoneda K, et al. Changes in lung function and health status in patients with COPD treated with tiotropium or salmeterol plus fluticasone. Respirology 2009;14:239-44. [PubMed]
- Mahler DA, Donohue JF, Barbee RA, et al. Efficacy of salmeterol xinafoate in the treatment of COPD. Chest 1999;115:957-65. [PubMed]
- O’Donnell DE, Voduc N, Fitzpatrick M, et al. Effect of salmeterol on the ventilatory response to exercise in chronic obstructive pulmonary disease. Eur Respir J 2004;24:86-94. [PubMed]
- Rennard SI, Anderson W, ZuWallack R, et al. Use of a long-acting inhaled beta2-adrenergic agonist, salmeterol xinafoate, in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2001;163:1087-92. [PubMed]
- Mochizuki H, Nanjo Y, Takahashi H. Better adherence to a transdermal tulobuterol patch than inhaled salmeterol in elderly chronic obstructive pulmonary disease patients. Geriatr Gerontol Int 2013;13:398-404. [PubMed]
- Fukuchi Y, Nagai A, Seyama K, et al. Clinical efficacy and safety of transdermal tulobuterol in the treatment of stable COPD: an open-label comparison with inhaled salmeterol. Treat Respir Med 2005;4:447-55. [PubMed]
- Teramoto S, Fukuchi Y, Orimo H. Effects of inhaled anticholinergic drug on dyspnea and gas exchange during exercise in patients with chronic obstructive pulmonary disease. Chest 1993;103:1774-82. [PubMed]
- Ainsworth BE, Haskell WL, Whitt MC, et al. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc 2000;32:S498-504. [PubMed]
- Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc 1982;14:377-81. [PubMed]
- Minami S, Kawayama T, Ichiki M, et al. Clinical efficacy of the transdermal tulobuterol patch in patients with chronic obstructive pulmonary disease: a comparison with slow-release theophylline. Intern Med 2008;47:503-9. [PubMed]
- Ichinose M, Seyama K, Nishimura M, et al. Additive effects of transdermal tulobuterol to inhaled tiotropium in patients with COPD. Respir Med 2010;104:267-74. [PubMed]
- Rau JL. Practical problems with aerosol therapy in COPD. Respir Care 2006;51:158-72. [PubMed]
- Gagnon P, Guenette JA, Langer D, et al. Pathogenesis of hyperinflation in chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis 2014;9:187-201. [PubMed]
- Guenette JA, Webb KA, O’Donnell DE. Does dynamic hyperinflation contribute to dyspnoea during exercise in patients with COPD? Eur Respir J 2012;40:322-9. [PubMed]