The impact of age on the survival outcomes and risk of radiation pneumonitis in patients with unresectable locally advanced non-small cell lung cancer receiving chemoradiotherapy
Introduction
The dynamic, chronological process of aging is characterized by the gradual accumulation of cell damage, progressive functional decline, and higher susceptibility and vulnerability to diseases (1). Elderly people are at increased risk of developing chronic diseases, including cancer, cardiovascular disease, hypertension, and diabetes mellitus.
With an estimated 2,093,876 new cases and 1,761,007 deaths in 2018, lung cancer is the most common and deadliest of any malignancy (2). Among elderly patients, the incidence and mortality of advanced lung cancer have been increasing (3). Previous studies have shown that the patient’s age at diagnosis plays an important role in the prognosis and treatment selection of lung cancer (4-6), prostate cancer (7,8), thyroid cancer (9), and lymphoma (10).
For patients with unresectable locally advanced non-small cell lung cancer (NSCLC), concurrent chemoradiotherapy (cCRT) followed by durvalumab can improve progression-free survival (PFS) [hazard ratio (HR) =0.52,95% confidence interval (CI): 0.42–0.65, P<0.001] and overall survival (OS) (HR =0.68, 95% CI: 0.53–0.87, P<0.001) compared with cCRT alone (11-13). However, cCRT carries the risk of developing radiation pneumonitis (RP). The sensitivity of lung to radiation remains the dose-limiting factor for radiotherapy for lung cancer. Severe RP is life threatening and RP also can delay or interrupt subsequent treatment and impact survival. In one study, a higher incidence of RP was shown in the Asian subgroup (14). It was important to evaluate the risk of RP and its relationship with age at diagnosis in Chinese population.
cCRT is still the important part of the multimodality therapy of PACIFIC pattern. Jeremic et al. (15) investigated the clinical prognostic factors in patients from Serbia with locally advanced NSCLC treated with chemoradiotherapy and the results showed only age did not influence OS and local PFS, whereas female gender, lower KPS, less pronounced weight loss, lower stage, squamous histology and treatment independently predicted better OS and local PFS. But the impact of age on survival in Chinese patients with unresectable locally advanced NSCLC receiving chemoradiotherapy was unknown. This study investigated the association of age with the survival outcomes and risk of RP in Chinese patients with unresectable locally advanced NSCLC receiving chemoradiotherapy from one institution. We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/jtd-20-2137).
Methods
Study population
The retrospective data of patients with unresectable locally advanced NSCLC who underwent radiotherapy (RT), sequential chemoradiotherapy (sCRT), or cCRT between January, 2013, to December, 2017, in our institution were reviewed. Inclusion criteria were as follows: age ≥18 years; obtained histologic confirmation of NSCLC; patients were IIIA and IIIB [7th edition TNM classification of the American Joint Committee on Cancer (AJCC)]; the patients, who received thoracic RT using IMRT or VMAT, form the population of this study. The patients with actual RT fractions less than 15, using split course RT, hypofractionation (single dose ≥2.5 Gy) and incomplete follow-up information were excluded from this population. The data of the patients were analyzed, including: age, gender, Karnofsky Performance Score (KPS), smoking history, histology, disease stage (7th edition of AJCC classification), radiation dose, chemotherapy, toxicity (RP, radiation esophagitis, skin reaction, and hematologic toxicity), disease progression, and survival time. Patients between January, 2013, to December, 2017 were included in this analysis to ensure enough sample size to avoid selection bias.
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Independent Ethics Committee of National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Science and Peking Union Medical College (No. 20/179-2375). Because of the retrospective nature of the research, the requirement for informed consent was waived.
Toxicity evaluation and follow-up
Toxicity was graded according to the Common Terminology Criteria for Adverse Events (CTC AE) version 4.0. Overall survival (OS) was measured from the date of initial treatment to the date of death from any cause, or to the time of last follow-up. Progression-free survival (PFS) was measured from the date of initial treatment to the date of disease progression or recurrence, or death from any cause. RP was defined as all RP (grades 1 to 5). Symptomatic RP was defined as RP ≥ grade 2. Severe RP was defined as RP ≥ grade 3. Fatal RP was classified as grade 5 RP.
Statistical analysis
A Cox proportional hazards model with restricted cubic spline (RCS) was used to investigate the relationship between age and OS. The optimal cutoff age was defined by maximally selected rank statistics from the 'maxstat' R package, which is an outcome-oriented method that provides a cut-point value with the most significant statistical relation with the outcome (16). The differences between groups were analyzed with Student’s t-test and χ2 test. Survival rates were calculated using the Kaplan-Meier method, and the survival outcomes of the groups were compared by log-rank test. A P value of <0.05 was considered statistically significant. Multivariate Cox regression analysis was conducted to determine the prognostic factors for survival outcomes and to calculate the hazards ratios (HRs). Analyses were performed using R software (Version 3.6.2) (http://www.r-project.org/). R packages included survival, survminer, rms, and maxstat.
Results
Optimal cutoff of age at diagnosis for OS
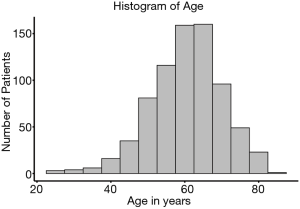
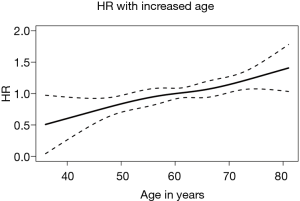
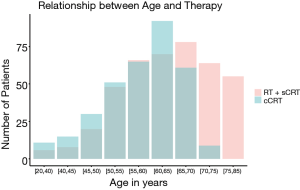
A database with 789 patients with unresectable locally advanced NSCLC who received RT in our institution was established. Ultimately, 749 patients with complete data available were included in the analysis. Of the 40 excluded patients, 37 patients were excluded due to missing disease progression information, 2 patients were excluded due to missing survival information, and 1 patient was excluded due to missing toxicity information. The age of the patients at diagnosis was normally distributed (median, 61 years; range, 23–83 years) (Figure 1). Figure 2 shows the HR of the patients’ age as a continuous variable by RCS (HR 1.18, 95% CI: 0.91–1.54, P<0.01). An age cutoff of 65 years was defined by maximally selected rank statistics. Based on this cutoff, the patients were stratified into two age groups: <65 years old (the younger group, n=482) and ≥65 years old (the older group, n=267).
Patient baseline characteristics and treatment
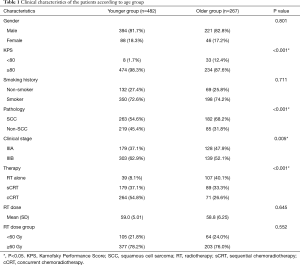
Of the patients, 615 (82.1%) patients were male, and 548 (73.2%) patients had a history of smoking. The majority (708, 94.5%) had good KPS (≥80). In terms of histology, 445 (59.4%) patients had squamous cell carcinoma (SCC) and 304 (40.6%) were non-SCC. According to the AJCC 7th edition staging classification, 307 (41.0%) patients were stage IIIA and 442 (59.2%) patients were stage IIIB. The baseline demographic and clinical characteristics of the two groups are listed in Table 1. There were more patients with poor KPS (<80) in the older group than in the younger group (12.4% vs. 1.7%, P<0.001). A higher proportion of patients in the older group had SCC (68.2% vs. 54.6%, P<0.001) and IIIA stage disease (47.9% vs. 37.1%, P=0.005). The older patients were more likely to have received RT alone (40.1% vs. 8.1%) and less likely to have received cCRT (26.6% vs. 54.8%) than the younger patients (P<0.001). As shown in Figure 3, the older patients received less cCRT than the younger patients. The radiation dose between the two groups was similar (P>0.05).
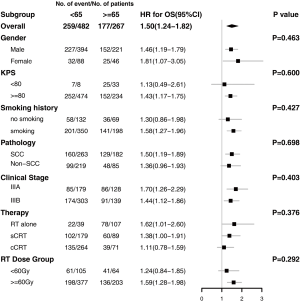
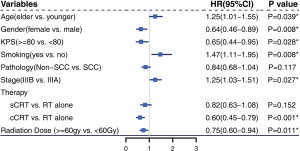
Full table
The prognostic value of age at diagnosis for OS
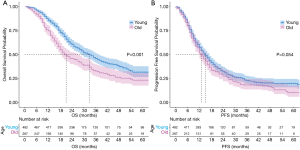
The median follow-up time was 22 months (range, 1–89 months) for the entire cohort, and 32 months (range, 1–83 months) in the surviving patients. A total of 259 deaths and 347 events of progression happened in younger group and 177 deaths and 198 events of progression happened in older group. The median OS was 33 months (95% CI: 29–37 months) and 21 months (95% CI: 18–27 months) in the younger group and older group, respectively (P<0.001) (Figure 4A). The 2- and 5-year OS rates were 60.3% and 31.6% in the younger group, respectively, and 45.9% and 23.2% in the older group, respectively. The median PFS was 15 months (95% CI: 13–17 months) in the younger group and 13 months (95% CI: 12–15 months) in the older group (P=0.054) (Figure 4B). The 2- and 5-year PFS rates were 32.5% and 19.7% in the younger group, respectively, and 27.5% and 10.3% in the older group, respectively. Multivariate Cox regression models revealed patient age at diagnosis to have a significant independent association with OS (HR: 1.25; 95% CI: 1.01–1.55) after covariate adjustment (gender, KPS, smoking, pathology, and disease stage) and treatment (therapy type and radiation dose) (Figure 5). In the subgroup analysis stratified by clinical factors (Figure 6), no source of heterogeneity was found. Patients >65 years old had high risk of death in male patients (HR =1.46, 95% CI: 1.19–1.79), female patients (HR =1.81, 95% CI: 1.07–3.05), patients with good KPS (HR =1.43, 95% CI: 1.17–1.75), patients with smoking history (HR =1.58, 95% CI: 1.27–1.96), patients with SCC (HR =1.50, 95% CI: 1.19–1.89), patients in IIIA (HR =1.70, 95% CI: 1.26–2.29) and IIIB stage (HR =1.44, 95% CI: 1.12–1.86), patients receiving RT alone (HR =1.62, 95% CI: 1.01–2.60) or sCRT (HR =1.38, 95% CI: 1.00–1.91), patients receiving RT dose of ≥60 Gy (HR =1.59, 95% CI: 1.28–1.98).
Toxicity
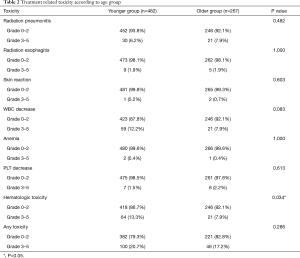
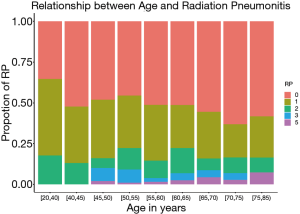
The radiation-related toxicities experienced by the patients are listed in Tables 2 and 3. The type and rate of toxicities were similar between the two groups. More patients suffered from grade 3–5 of hematologic toxicity in the younger group than in the older group (13.3% vs. 7.9%, P=0.034). Although the incidences of symptomatic RP and severe RP were similar in the two groups (18.9% and 6.2% in the younger group vs. 16.1% and 7.9% in the older group, P<0.05). There was a higher incidence of fatal RP in the older group (4.5% vs. 1.7%, P=0.039). As shown in Figure 7, the incidence of fatal RP increased with age.
Full table

Full table
Discussion
In this study, we found that the clinical characteristics of elderly patients (≥65 years old) and young patients differed. Older patients were also more likely to choose conservative treatment. OS was higher in the younger patients in our study than in the older group. We also found a higher incidence of fatal RP in the older group. To our knowledge, this is the first report about the relationship between fatal RP and age.
Table 1 shows that 12.4% of the older group had poor performance status (KPS <80), compared with 1.7% of the younger group (P<0.001), and that SCC was more common in the older group than the younger group (68.2% vs. 54.6%, P<0.001). Data from the National Cancer Data Base (NCDB) (4) showed similar results. In this analysis, only stage III NSCLC patients were included, and the younger group had more stage IIIB cases (62.9% vs. 52.1%, P=0.004). NCDB (4) and SEER (5) analysis involving all stages of NSCLC found more cases of stage IV lung cancer in younger patients. These analyses and our data showed that younger patients tended to have more aggressive, late-stage tumors. Consistent with our findings, previous data showed that younger patients were more likely to receive the recommended treatment, while older patients were more likely to choose no treatment or conservative treatment (4,5). Of the older patients in our study, we found that only 26.6% received cCRT and 40.1% received RT alone. The proportion of older patients who received RT alone was higher than that of younger patients.
Younger patients generally have better prognosis than older patients; this has been demonstrated in studies on prostate cancer (7,8), thyroid cancer (9), and lymphoma (10). Similar results were also observed for NSCLC in NCDB (4) and SEER (5,6) analysis. Our analysis focused specifically on patients with unresectable locally advanced NSCLC who received either RT alone, sCRT, or cCRT. Our conclusion was consistent with the results of these previous studies. Multivariate Cox regression analysis (Figure 5) determined age at the time of diagnosis to be an independent prognostic factor for OS. Subgroup analysis produced the same results (Figure 6). Figure 4A,B showed that the younger patients had better OS than the older patients, while the two groups had similar PFS. The NCDB (4) and SEER (5,6) studies did not take PFS data or the relationship between PFS and OS into account. We hypothesized that the following reasons may be related to the poorer OS of the older patients and the similar PFS of the two groups. First, the older patients were more likely to refuse recommended therapy after progression. Second, the older patients had a higher risk of dying from causes other than lung cancer (analysis from SEER database, which was not reported yet). More data are needed to approve these two hypotheses. Third, as shown in Table 3 and Figure 7, the rate of fatal RP was higher in the older patients than in the younger patients, while the rates of symptomatic RP and other toxicities were similar in both groups. Some studies have found that elderly patients are at increased risk of side effects from chemotherapy or targeted therapy (17,18). Our analysis did not identify this trend in radiation esophagitis, skin reaction, and hematologic toxicity, which may be related to fewer patients in the older group receiving chemoradiotherapy. Palma et al. (19) found that the incidence of symptomatic RP increased with age in univariate analysis; however, this was not reflected in multivariate analysis. Meanwhile, Tsujino (20) found that older patients were more likely to have severe RP (20.6% vs. 8.0%, P=0.05). Neither of these two studies reported the relationship between age and fatal RP. We found higher incidences of fatal RP and similar symptomatic RP in the older group after RT than in the younger group, which means the elderly patients were less likely to recover from symptomatic RP.
This study has some limitations. First, this analysis was based on a retrospective, single-institution database. Also, compared with the SEER and NCDB studies, which involved hundreds of thousands of individuals, only 749 patients were enrolled in our study (3,4,6). Furthermore, some relevant information, such as the treatment compliance, treatment after progression, and cause of death, was missing. This information may help to explain how age affects patients’ survival. The NCDB study used modified Charlson-Deyo scores to record the comorbidity of patients and found that those with a higher age had a higher score; however, this was not available in our analysis. Additionally, patients with incomplete information or those lost to follow-up were excluded, which may have led to bias. After the amazing results of the PACIFIC trail were published (11,12), durvalumab as consolidation therapy was recommended for unresectable stage III NSCLC. Although the patients included in our data were treated before the PACIFIC era and no patients received consolidative immunotherapy, the current analysis stresses the need to pay more attention to elderly patients when consolidative immunotherapy is used, due to the high risk of fatal RP in older patients.
In conclusion, the elderly patients with unresectable locally advanced NSCLC in our study had poorer performance status and were more likely to have SCC and stage IIIA disease than younger patients. They also tended to choose conservative treatment more. Importantly, age at diagnosis had prognostic value for OS. More attention should be paid to the risk of fatal RP associated with chemoradiotherapy with/without consolidative immunotherapy in elderly patients.
Acknowledgments
Funding: This work was supported by CAMS Innovation Fund for Medical Sciences (2017-I2M-1-009).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/jtd-20-2137
Data Sharing Statement: Available at http://dx.doi.org/10.21037/jtd-20-2137
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd-20-2137). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Independent Ethics Committee of National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Science and Peking Union Medical College (No. 20/179-2375). Because of the retrospective nature of the research, the requirement for informed consent was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Luo J, Mills K, le Cessie S, et al. Ageing, age-related diseases and oxidative stress: What to do next? Ageing Res Rev 2020;57:100982. [Crossref] [PubMed]
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Owonikoko TK, Ragin CC, Belani CP, et al. Lung cancer in elderly patients: an analysis of the surveillance, epidemiology, and end results database. J Clin Oncol 2007;25:5570-7. [Crossref] [PubMed]
- Tang W, Lei Y, Su J, et al. TNM stages inversely correlate with the age at diagnosis in ALK-positive lung cancer. Transl Lung Cancer Res 2019;8:144-54. [Crossref] [PubMed]
- Subramanian J, Morgensztern D, Goodgame B, et al. Distinctive characteristics of non-small cell lung cancer (NSCLC) in the young: a surveillance, epidemiology, and end results (SEER) analysis. J Thorac Oncol 2010;5:23-8. [Crossref] [PubMed]
- Chen T, Zhou F, Jiang W, et al. Age at diagnosis is a heterogeneous factor for non-small cell lung cancer patients. J Thorac Dis 2019;11:2251-66. [Crossref] [PubMed]
- Pettersson A, Robinson D, Garmo H, et al. Age at diagnosis and prostate cancer treatment and prognosis: a population-based cohort study. Ann Oncol 2018;29:377-85. [Crossref] [PubMed]
- Bechis SK, Carroll PR, Cooperberg MR. Impact of age at diagnosis on prostate cancer treatment and survival. J Clin Oncol 2011;29:235-41. [Crossref] [PubMed]
- Adam MA, Thomas S, Hyslop T, et al. Exploring the Relationship Between Patient Age and Cancer-Specific Survival in Papillary Thyroid Cancer: Rethinking Current Staging Systems. J Clin Oncol 2016;34:4415-20. [Crossref] [PubMed]
- Liu WX, Shi M, Su H, et al. Effect of age as a continuous variable on survival outcomes and treatment selection in patients with extranodal nasal-type NK/T-cell lymphoma from the China Lymphoma Collaborative Group (CLCG). Aging (Albany NY) 2019;11:8463-73. [Crossref] [PubMed]
- Antonia SJ, Villegas A, Daniel D, et al. Overall Survival with Durvalumab after Chemoradiotherapy in Stage III NSCLC. N Engl J Med 2018;379:2342-50. [Crossref] [PubMed]
- Antonia SJ, Villegas A, Daniel D, et al. Durvalumab after Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. N Engl J Med 2017;377:1919-29. [Crossref] [PubMed]
- Gray JE, Villegas A, Daniel D, et al. Three-Year Overall Survival with Durvalumab after Chemoradiotherapy in Stage III NSCLC-Update from PACIFIC. J Thorac Oncol 2020;15:288-93. [Crossref] [PubMed]
- Vansteenkiste J, Naidoo J, Faivre-Finn C, et al. PACIFIC Subgroup Analysis: Pneumonitis in Stage III, Unresectable NSCLC Patients Treated with Durvalumab vs. Placebo After CRT. J Thorac Oncol 2018;13:S370-1. [Crossref]
- Jeremic B, Milicic B, Milisavljevic S. Clinical prognostic factors in patients with locally advanced (stage III) nonsmall cell lung cancer treated with hyperfractionated radiation therapy with and without concurrent chemotherapy: single-Institution Experience in 600 Patients. Cancer 2011;117:2995-3003. [Crossref] [PubMed]
- Hothorn T, Lausen B. On the exact distribution of maximally selected rank statistics. Computational Statistics & Data Analysis 2003;43:121-37. [Crossref]
- Sederholm C, Hillerdal G, Lamberg K, et al. Phase III trial of gemcitabine plus carboplatin versus single-agent gemcitabine in the treatment of locally advanced or metastatic non-small-cell lung cancer: the Swedish Lung Cancer Study Group. J Clin Oncol 2005;23:8380-8. [Crossref] [PubMed]
- Wheatley-Price P, Ding K, Seymour L, et al. Erlotinib for advanced non-small-cell lung cancer in the elderly: an analysis of the National Cancer Institute of Canada Clinical Trials Group Study BR.21. J Clin Oncol 2008;26:2350-7. [Crossref] [PubMed]
- Palma DA, Senan S, Tsujino K, et al. Predicting radiation pneumonitis after chemoradiation therapy for lung cancer: an international individual patient data meta-analysis. Int J Radiat Oncol Biol Phys 2013;85:444-50. [Crossref] [PubMed]
- Tsujino K, Hashimoto T, Shimada T, et al. Combined analysis of V20, VS5, pulmonary fibrosis score on baseline computed tomography, and patient age improves prediction of severe radiation pneumonitis after concurrent chemoradiotherapy for locally advanced non-small-cell lung cancer. J Thorac Oncol 2014;9:983-90. [Crossref] [PubMed]
(English Language Editor: J. Reynolds)