
Is invasive mediastinal staging necessary in intermediate risk patients with negative PET/CT?
Introduction
Surgery is the best therapeutic option for early stage non-small cell lung cancer (NSCLC). However, it is not recommended if mediastinal lymph node are involved (1). Therefore, mediastinal lymph node staging is key for NSCLC management (2). In the absence of mediastinal involvement, resectable tumors can be surgically treated, whereas patients with mediastinal affection (cN2 or cN3) are initially excluded from surgery and treated with radical chemo-radiotherapy (3).
Patients with non-bulky (<2 cm) single positive mediastinal station detected preoperatively (cN2) can be treated with neo-adjuvant chemo(radio) therapy followed by surgery, or surgery followed by chemo(radio) therapy (4). Positron Emission Tomography - Computed Tomography with 18F-Fluordeoxyglucose (FDG PET/CT) is used to detect cN2 (5), but there is a subgroup of patients (central tumors, tumors larger than 3 cm, cN1 patients) with higher risk of pN2 despite negative image staging in whom invasive mediastinal staging by endobronchial ultrasound (EBUS) and/or Video-assisted Mediastinoscopy (VAM) is recommended by international societies and included in the guidelines (6).
This retrospective analysis of all patients treated surgically for NSCLC at our institution between 2013 and 2018 sought to: (I) evaluate the effectiveness of invasive mediastinal staging to reduce unexpected pN2 incidence in intermediate-risk patients and its impact on survival; and, (II) determine the risk factors for occult pN2.
We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/jtd-20-1248).
Methods
Study design, patients and ethics
All patients treated for NSCLC in our institution between 2013 and 2018 were prospectively included in the analysis when no mediastinal affection was detected by CT or PET/CT and fulfilled one of the following criteria: NSCLC tumors larger than 3 cm by CT scan, tumors considered central or cN1 cases by CT or PET/CT.
Patients who had received previous lung cancer treatment, not suitable for surgery, with unresectable pathology or suspicion of cN2-3 or cM1 were excluded from the analysis. In case of two synchronous tumors, the largest one was taken in consideration for analysis. Patients were clinically (cTNM) and pathologically (pTNM) staged according to the 8th edition of the international TNM classification (7). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). All participants had signed conventional informed consent before any staging exploration or surgery. The Ethics Committee of our institution approved the study protocol.
Mediastinal involvement terminology
Four scenarios referring mediastinal lymph node involvement are used in this study and described:
❖ Clinical mediastinal involvement (cN2): used in those cases in which histologic diagnosis was obtained before surgery either by EBUS or VATS.
❖ Pathological mediastinal involvement (pN2) is used when histologic diagnosis is obtained after surgical lymphadenectomy. It includes patients with previously known affection (cN2) who had induction treatment and surgery.
❖ Any mediastinal involvement (N2) is used referring to all patients who had mediastinal involvement at any moment regardless of whether they underwent surgery (N2= cN2 + pN2).
Preoperative Image staging
Pre-operative lung cancer staging was done according to international recommendations. All patients were initially staged using CT and PET/CT. cN1 was defined by CT when hilar lymph nodes >10 mm in short axis or by FDG-PET/CT when lymph node uptake was higher than the surrounding mediastinal tissue. Centrality was defined as tumor growing in the inner third of the chest in a dedicated CT scan (8). Tumor size was also recorded.
Invasive mediastinal staging
When considered necessary by the multi-disciplinary lung cancer committee of our institution, presurgical invasive mediastinal staging was done using EBUS and/or VAM.
Endobronchial ultrasound
EBUS were done by two teams in two different centers, Hospital Clínic de Barcelona and Hospital de Mollet. EBUS were performed under sedation with a continuous infusion of propofol and remifentanil, maintaining spontaneous breathing in the bronchoscopy unit by a staff anesthesiologist. The bronchoscopist inserted the scope through mouth and lidocaine was used as topical airway anaesthesia. EBUS-TBNA was performed using a flexible ultrasound bronchoscope (Pentax EB-1970UK 2.0) and 22-gauge needle (Echotip® Ultra, Cook Medical, US). All identified nodes with a minor diameter of ≥5 mm were sampled (6). The sampling site was selected according to clinical guidelines. The first sampled area was N3 followed by N2 and ending with N1 nodes. The cytology preparation was performed with both air-dried and wet-fixed methods. A rapid on-site evaluation (ROSE) was done by an experienced cytotechnologist to ensure the sample quality and to identify tumor cells. Lymph node was considered no malignant after 3 passes showing normal lymphocytes with no malignant cells. The presence of diagnostic malignant material led to the completion of the procedure. Any aspirate was placed in formaldehyde solution to cell block preparation. After an observational period patients were discharged.
Video-assisted mediastinoscopy (VAM)
All VAMs were carried out in a dedicated operating room by one of the five staff-thoracic surgeons from the Hospital Clínic department. VAM was performed under general anesthesia through a transverse cervicotomy until the opening of pretracheal fascia. After finger blunt dissection the video-mediastinoscope was introduced into the mediastinum and exploration of both paratracheal and subcarinal spaces (2R, 4R, 4L and 7) was performed in all cases as recommended (9). When detected, lymph nodes were biopsied individually. Deferred pathological assessment was done using hematoxylin-eosin staining and immunohistochemistry.
Positive staging patients (cN2)
All patients diagnosed with cN2 after invasive mediastinal staging were discussed in the multidisciplinary oncologic board. Those with contralateral lymph node involvement (cN3), multi-station ipsilateral involvement (cN2 multi), single level ipsilateral lymph node involvement >3 cm (cN2 bulky) and infiltrative non-resectable pN2 were discarded for surgical treatment and excluded from the study. Single level ipsilateral non-bulky N2 affection, had neoadjuvant treatment (chemotherapy or chemo-radiotherapy) followed by surgical resection if no progression was detected after being reassessed by CT.
Surgery
Patients with no mediastinal affection and those whom completed induction therapy were operated. All surgeries were performed by one of the five experienced thoracic surgeons from Hospital Clinic’s department. Anatomic lung resection was performed in all patients (segmentectomy to pneumonectomy) either through open or thoracoscopic approach. Lung sparing techniques and extended resections were carried out whenever considered necessary in order to achieve complete resection. In addition, all patients had a systematic mediastinal lymph node dissection including stations 5 and 6 in left sided tumors (10). Incidence of postoperative mediastinal lymph node affection in those patients considered cN0-1 (pN2) and incidence of all histologically confirmed N2 after surgery (cN2 + pN2) were recorded.
Postoperative treatment and follow up
Patients were treated with chemotherapy and/or radiotherapy following the international guidelines (3,4). All patients were followed up periodically in referral center’s outpatient clinic.
Statistical analysis
Results are presented as n (proportion), mean ± standard deviation or median [interquartile range] as appropriate. The invasive staging and non-invasive staging groups were compared using Fisher’s exact test, Chi-Square, Student’s t-test or ANOVA as appropriate. Multivariate (logistic regression) correlation analysis were used to investigate risk factors of occult pN2. Kaplan-Meier curves were used to compare overall survival between staged and non-staged groups and between staged and non-staged pN2. Cox hazard model was used to evaluate relation among invasive staging and survival. A P value <0.05 was considered statistically significant. All analyses were done using SPSS 20.0 (IBM Corporation, Armonk, NY, USA).
Results
Patients characteristics
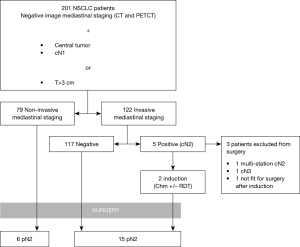
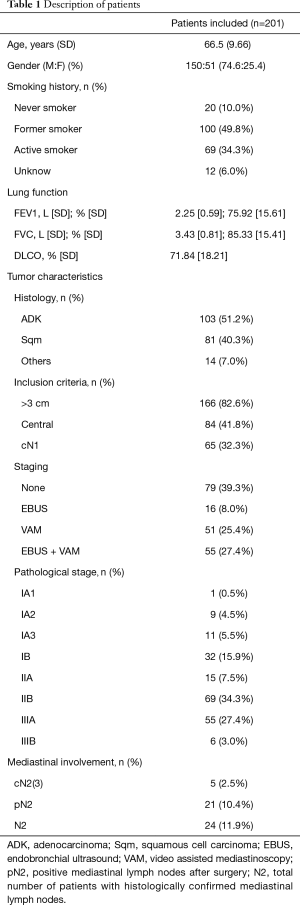
During the study period 201 patients were included for analysis (Figure 1). Their general characteristics are summarized in Table 1. The most common inclusion criteria in our study was tumor size (82.6%), followed by centrality (41.8%) and cN1 (32.3%). Invasive staging was carried out in 60.7% of cases either by EBUS alone (8%), EBUS+VAM (27.4%) or VAM alone (25.4%). During VAM procedure 2.9±0.83 mediastinal stations were biopsied. Mean time between last mediastinal exploration (EBUS or VAM) and surgery was 26.9±15.24 days. Global invasive staging negative predictive value (NPV) in our series of patients was 0.974. Lymphadenectomy was performed to all surgical patients exploring 3.63±1.13 stations, including paratracheal, subcarinal, lower mediastinal and aorto-pulmonary window (left side tumors). Mean follow up was 25.31±18 months.
Full table
Some of the patients included in the present series had previously participated in different clinical protocols. In particular 16 patients with suspected lung cancer and evidence of cN1 by CT scan and/or PET had been included in the ASTER 3 (Assessment of Surgical Staging vs. Endosonographic Ultrasound in Lung Cancer: a Randomized Clinical Trial) study that assessed the usefulness of straightforward mediastinoscopy in this setting. The results of this study have been published elsewhere (11). Similarly, 49 patients of the present series were invasively staged systematically using EBUS followed by VAM (if EBUS negative) and included in a prospective evaluation aimed to assess usefulness of EBUS (performed in our institution) for mediastinal evaluation in selected patients. This study has currently finished the recruitment of patients and the results are being processed for a forthcoming manuscript.
Invasive staging vs. non-invasive staging
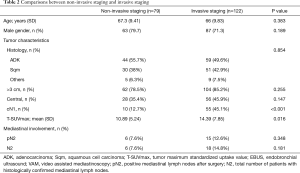
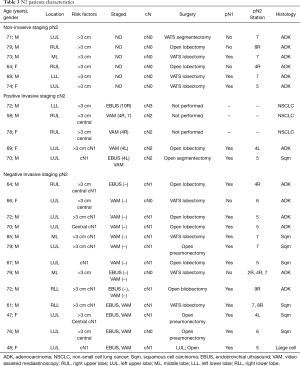
When IS and NIS groups are compared (Table 2) the only two differences between groups are cN1 incidence, that is higher in invasive staging group (12.7% vs. 45.1%, P<0.001), and mean PET/CT SUVmax (10.89 in NIS group vs. 14.39 in IS group; P=0.016). Mean tumor size was smaller in NIS group (4.25 cm±1.93) compared to IS group (4.9cm±2.12) (P=0.017) but there was no difference in the incidence of tumors >3 cm (78.5% vs. 85.2%; P=0.255). Postoperative mediastinal lymph nodes affection (pN2) was detected in 6 cases (7.6%) in non-invasive staging group and 15 cases (13.3%) [including 2 preoperatively detected who had induction therapy (cN2)] in invasive staging group (P=0.348). Table 3 details the characteristics of patients with mediastinal lymph node affection (N2).
Full table
Full table
When pN2 patients are analyzed 10 out of 21 patients (47.6%) had positive lymph nodes after surgery in a non-reachable station by conventional EBUS and VAM. This includes pulmonary window (stations 5 and 6) in 7 cases (33.3%) and lower mediastinal stations (station 8 and 9) in 3 cases (14.3%). Finally, 2 patients (2/21; 9.5%) had multistation mediastinal involvement at surgery, all of them at NIS group.
Risk factors for pN2
Multivariate analysis (Table 4) identified cN1 as the only risk factor for pN2 patients (P=0.013). Invasive mediastinal staging did not emerge as an independent risk factor for unexpected pN2 (P=0.583).
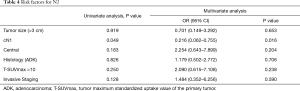
Full table
Survival
Figure 2 shows Kaplan-Meier curves for overall survival among IS vs. NIS groups. Median survival was lower for those patients with any pN2-3 (33.4 months) when compared with pN0-1 patients (46.4 months) (P=0.034). No statistical differences where detected when invasive staging and non-invasive staging groups were compared (40.3 vs. 45.6 months; P=0.721). In pN2 subgroup, median survival was 34.7 months in IS and 22.5 months in NIS (P=0.152).
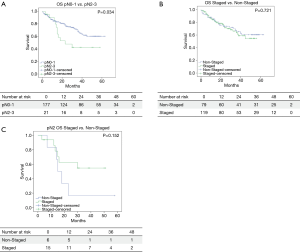
Survival Cox Hazard model (Table 5) showed no direct relation among staging and survival when all patients were compared.
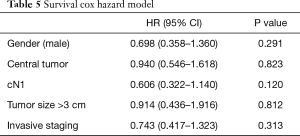
Full table
Discussion
This study shows that, in our series, there are no differences regarding N2 and pN2 incidence between invasively staged and non-invasively staged group. Preoperative hilar lymph node tumor involvement (cN1) was identified as the only risk factor for postoperative mediastinal lymph node affection (pN2). Survival in pN2 patients was significantly worse than in pN0-1 patient. Differences in survival between invasively staged pN2 and non-invasively staged pN2 are not statistically significant but they are clinically relevant.
In our study, postoperative confirmed mediastinal lymph node affection (pN2) was detected in 21 cases (21/201; 10.4%). This is comparable to c-stage I NSCLC incidence published by other groups (12) in accordance with international recommendations and European Society of Thoracic Surgery (ESTS) guidelines (6). Mediastinal involvement (N2) on invasively staged group was detected on 18 patients, 5 out these 18 (27.8%) histologically confirmed N2 patients were identified preoperatively (cN2) in our series. The incidence of pN2 among IS and NIS groups was statistically non significant (7.6% vs. 12.6%, P=0.348).
Multivariate analysis determined cN1 as the only risk factor for pN2. Other papers described an increased incidence of mediastinal unexpected lymph nodes reaching 25–37% when cN1 is present (11,13). Contrarily to other studies tumor size and location are not independent risk factors in our group of patients. Our previously published work from 2012 showed that tumor size is an independent risk factor for upstaging; however, we analyzed only clinical stage I NSCLC and excluded central tumors (14). The same occurs in other papers where the analysis is done only in cN0 patients (12,15). Concerning to tumor location, we did not identify centrality as an independent risk factor for pN2. Belgian group published results in the same direction putting in doubt the necessity of invasive mediastinal staging in this subgroup of patients (16). It is true, however, that tumors located in the inner third of the chest have an increased risk of hilar lymph node upstaging (pN1) as reported in a multicentric study (17) and recently published by our group (18). In summary, our findings support the notion that only cN1 status should be considered an independent risk factor for pN2. Tumor size and location are risk factors for pN1 and could be indirect risk factors for pN2.
To determine whether it is worthwhile to perform systematic invasive mediastinal staging in intermediate risk patients or not, therapeutic strategy should be decided first. Based on the first randomized trials published by Roth and Rosell et al. (19,20), cN2 patients could benefit from surgical treatment after induction surgery when complete resection is achieved and pneumonectomy avoided. Other groups advocate for straight surgery in single station non bulky cN2 based in the absence of differences on survival whether chemotherapy is administered before or after surgery (21). They also determine that potentially surgical complications related to induction treatment could be avoided. In our center, the multidisciplinary oncological tumor board agreed that single station non-bulky cN2 patients are treated with chemotherapy (± radiotherapy) before surgery. Poor prognosis of pN2 (22) added to this scenario makes advisable to invasively stage those patients with intermediate risk factors to identify cN2. However, considering the results of the current study, invasive efforts may not lead to a reduction in incidental N2.
Based on these results, should we go straight to surgery as some groups prefer (23)? Probably not, but as Cerfolio et al. published, better selection means better survival (24). Looking deeper into our series of patients, 3 out of 5 identified cN2 patients were discarded for surgery because multi-station involvement, cN3 and poor tolerance to induction therapy. Despite survival among invasively staged and non-invasively staged pN2 groups is statistically non-significant, mean survival time increase in 11 months in invasive staging group (33.6 vs. 22.5 months; P=0.245) leads us to consider it as clinically relevant. These differences might be explained by better patient selection and treatment. They are concordant with results previously published by Obiols et al. (25).
Whether invasive staging should be done by EBUS or VAM is another topic on debate (26). EBUS is a less invasive technique for the patient and does not require hospital stay with the cost savings it represents. In our experience VAM was not able to identify cN2 when EBUS was negative (Table 3). In the case of cN1, VAM as first exploration or after EBUS is recommendable based on a prospective multicentric study published in 2017 (11). Our study cannot conclude which technique is better because EBUS results are dependent on the technique and group experience, so further studies are necessary in this specific patient category. Finally, half of the pN2 patients were not reachable by EBUS or VAM and 36.8% of them were located in stations 5 and 6. In our future practice extended VAM or VATS should be added in cN1 left lung carcinomas as other groups recommended (27).
Study limitations
First, this is not a randomized study. The higher incidence of cN1 in invasive staging group is related to participation in other studies during the same period and clinical criteria. Since cN1 is the only risk factor for mediastinal involvement, prevalence of N2 could be influenced.
Second, we analyzed the impact of invasive mediastinal staging on pN2 and survival instead of which technique to use because EBUS were performed in two different centers (Hospital Clínic and Hospital de Mollet). EBUS sensitivity and specificity are technical dependent and they are related to experience. Hospital Clínic's EBUS sensitivity and specificity are being analyzed and future conclusions will help us to determine the best staging strategy.
Conclusions
In our series of intermediate-risk patients, (A) there was no statistical difference in pN2 incidence between the invasively staged and non-invasively staged groups. However it could be attributed to asymmetric distribution of cN1 patients that is indeed (B) the only independent risk factor for pN2.
Despite this absence of statistical differences among pN2 incidence better patient selection might have an impact on survival. In conclusion, it all makes invasive staging recommendable when intermediate risk patients for mediastinal lymph node involvement are evaluated for surgery.
Acknowledgments
Authors thank the Hospital Clinic’s Thoracic Oncology Unit and affiliate hospitals, as well to the anesthesiology, critical care and ward team (doctors and nurses) for their daily work. Special thanks to Dr. Àlvar Agustí for his comments and support and Francesc Gahete for his patience and advice.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/jtd-20-1248
Peer Review file: Available at http://dx.doi.org/10.21037/jtd-20-1248
Data Sharing Statement: Available at http://dx.doi.org/10.21037/jtd-20-1248
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd-20-1248). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional ethics committee of Hospital Clínic de Barcelona (NO. HCB/2019/0435) and informed consent was taken from all the patient.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Asamura H, Chansky K, Crowley J, et al. The International Association for the Study of Lung Cancer Lung Cancer Staging Project. J Thorac Oncol 2015;10:1675-84. [Crossref] [PubMed]
- Silvestri GA, Gonzalez AV, Jantz MA, et al. Methods for Staging Non-small Cell Lung Cancer: Diagnosis and Management of Lung Cancer, 3rd ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2013;143:e211S-e250S.
- Detterbeck FC, Lewis SZ, Diekemper R, et al. Diagnosis and Management of Lung Cancer, 3rd ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines Chest 2013;143:7S-37S.
- Postmus PE, Kerr KM, Oudkerk M, et al. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2017;28:iv1-21. [Crossref]
- Al-Jahdali H, Khan AN, Loutfi S, et al. Guidelines for the role of FDG-PET/CT in lung cancer management. J Infect Public Health 2012;5:S35-40. [Crossref] [PubMed]
- De Leyn P, Dooms C, Kuzdzal J, et al. Revised ESTS guidelines for preoperative mediastinal lymph node staging for non-small-cell lung cancer. Eur J Cardiothorac Surg 2014;45:787-98. [Crossref] [PubMed]
- Rami-Porta R. Staging Manual in Thoracic Oncology 2nd Ed. North Fort Myers, FL; 2016.
- Sanchez-Lorente D, Milla L, Guirao A, et al. MA10.06 Centrality definition in non-small cell lung cancer. predictor for occult mediastinal lymph node involvement. J Thorac Oncol 2017;12:S400. [Crossref]
- De Leyn P, Dooms C, Kuzdzal J, et al. Preoperative mediastinal lymph node staging for non-small cell lung cancer: 2014 update of the 2007 ESTS guidelines. Transl Lung Cancer Res 2014;3:225-33. [PubMed]
- Lardinois D, De Leyn P, Vanschil P, et al. ESTS guidelines for intraoperative lymph node staging in non-small cell lung cancer. Eur J Cardiothorac Surg 2006;30:787-92. [Crossref] [PubMed]
- Decaluwé H, Dooms C, D’Journo XB, et al. Mediastinal staging by videomediastinoscopy in clinical N1 non-small cell lung cancer: a prospective multicentre study. Eur Respir J 2017;50:1701493. [Crossref] [PubMed]
- Fiorelli A, Sagan D, Mackiewicz L, et al. Incidence, risk factors, and analysis of survival of unexpected N2 disease in stage I non-small cell lung cancer. Thorac Cardiovasc Surg 2015;63:558-67. [Crossref] [PubMed]
- Watanabe S, Asamura H, Suzuki K, et al. Problems in diagnosis and surgical management of clinical N1 non-small cell lung cancer. Ann Thorac Surg 2005;79:1682-5. [Crossref] [PubMed]
- Gómez-Caro A, Boada M, Cabañas M, et al. False-negative rate after positron emission tomography/computer tomography scan for mediastinal staging in ci stage non-small-cell lung cancer. Eur J Cardiothorac Surg 2012;42:93-100; discussion 100. [Crossref] [PubMed]
- Koike T, Yamato Y, Yoshiya K, et al. Predictive Risk Factors for Mediastinal Lymph Node Metastasis in Clinical Stage IA Non–Small-Cell Lung Cancer Patients. J Thorac Oncol 2012;7:1246-51. [Crossref] [PubMed]
- Decaluwé H, Moons J, Fieuws S, et al. Is central lung tumour location really predictive for occult mediastinal nodal disease in (suspected) non-small-cell lung cancer staged cN0 on 18F-fluorodeoxyglucose positron emission tomography-computed tomography? Eur J Cardiothorac Surg 2018;54:134-40. [Crossref] [PubMed]
- Decaluwé H, Petersen RH, Brunelli A, et al. Multicentric evaluation of the impact of central tumour location when comparing rates of N1 upstaging in patients undergoing video-assisted and open surgery for clinical Stage I non-small-cell lung cancer†. Eur J Cardiothorac Surg 2018;53:359-65. [Crossref] [PubMed]
- Boada M, Guzmán R, Montesinos M, et al. Upstaging, centrality and survival in early stage non-small cell lung cancer video-assisted surgery. Lung Cancer 2019;134:254-8. [Crossref] [PubMed]
- Roth JA, Fossella F, Komaki R, et al. A randomized trial comparing perioperative chemotherapy and surgery with surgery alone in resectable stage IIIA non-small-cell lung cancer. J Natl Cancer Inst 1994;86:673-80. [Crossref] [PubMed]
- Rosell R, Gomez-Codina J, Camps C, et al. A Randomized Trial Comparing Preoperative Chemotherapy Plus Surgery with Surgery Alone in Patients with Non-Small-Cell Lung Cancer. N Engl J Med 1994;330:153-8. [Crossref] [PubMed]
- Zheng D, Ye T, Hu H, et al. Upfront surgery as first-line therapy in selected patients with stage IIIA non–small cell lung cancer. J Thorac Cardiovasc Surg 2018;155:1814-1822.e4. [Crossref] [PubMed]
- Suzuki K, Nagai K, Yoshida J, et al. The prognosis of surgically resected N2 non–small cell lung cancer: The importance of clinical N status. J Thorac Cardiovasc Surg 1999;118:145-53. [Crossref] [PubMed]
- Regnard J, Magdeleinat P, Azoulay D, et al. Results of resection for bronchogenic carcinoma with mediastinal lymph node metastases in selected patients. Eur J Cardiothorac Surg 1991;5:583-7. [Crossref] [PubMed]
- Cerfolio RJ, Maniscalco L, Bryant AS. The Treatment of Patients with Stage IIIA Non-Small Cell Lung Cancer From N2 Disease: Who Returns to the Surgical Arena and Who Survives. Ann Thorac Surg 2008;86:912-20. [Crossref] [PubMed]
- Obiols C, Call S, Rami-Porta R, et al. Survival of Patients With Unsuspected pN2 Non-Small Cell Lung Cancer After an Accurate Preoperative Mediastinal Staging. Ann Thorac Surg 2014;97:957-64. [Crossref] [PubMed]
- Hegde PV, Liberman M. Mediastinal Staging: Endosonographic Ultrasound Lymph Node Biopsy or Mediastinoscopy. Thorac Surg Clin 2016;26:243-9. [Crossref] [PubMed]
- Call S, Rami-Porta R, Serra-Mitjans M, et al. Extended cervical mediastinoscopy in the staging of bronchogenic carcinoma of the left lung. Eur J Cardiothorac Surg 2008;34:1081-4. [Crossref] [PubMed]


