Elevated expression of USP9X correlates with poor prognosis in human non-small cell lung cancer
Introduction
Lung cancer is the most frequent malignancy and is the leading cause of cancer-related death worldwide (1). Non-small cell lung cancer (NSCLC) accounts for greater than 80% of all lung cancer types (2). In spite of recent advances in surgical techniques and in various strategies of chemotherapy and radiotherapy, the 5-year survival rate of patients with NSCLC is only approximately 15% (3). Moreover, due to the mild symptoms at the early stage and the difficulty in making an early diagnosis, most patients with NSCLC are diagnosed at an advanced stage. Therefore, it is necessary to identify molecular makers that enable early diagnosis and the accurate prediction of prognosis.
In recent years, ubiquitination has been demonstrated as a versatile post-translational modification that regulates various aspects of cellular physiology via the activity of ubiquitylating and deubiquitylating enzymes (DUBs) (4,5). More attention has been drawn to DUBs due to their wide functional diversity, including their involvement in cell-cycle control, DNA repair and several signaling pathways that are frequently altered during tumor progression (6,7). Ubiquitin-specific peptidases (USPs), the largest group of DUBs, play fundamental roles in the ubiquitin system by specifically deconjugating ubiquitin from ubiquitylated substrates. ubiquitin-specific peptidase 9, X-linked (USP9X), a member of the USP family, is involved in multiple physiological pathways by targeting a variety of substrates. Elevated expression of USP9X at the translational level has been shown to correlate with poor prognosis in multiple myeloma and esophageal squamous cell carcinoma (8,9). Furthermore, evidence suggests that USP9X can stabilize MCL1, a member of the pro-survival BCL-2 family, to promote cell survival. Alternatively, the knockdown of USP9X expression induces MCL1 turnover and cell death by increasing MCL1 polyubiquitination (9).
Despite the numerous efforts to investigate the function of the ubiquitin hydrolase USP9X, the correlation between USP9X expression and survival or prognosis among NSCLC patients is largely unknown. This study was mainly to investigate USP9X expression and its clinical significance in NSCLC and normal lung tissues by immunohistochemistry method.
Materials and methods
Patients and tissue samples
Paraffin-embedded postoperative tissue samples from 95 patients with clinical stage I-IIIA NSCLC were collected from the archives of the Department of Pathology at Zhongnan Hospital of Wuhan University between July 2008 and July 2011. Among these 95 patients, only 32 patients exhibited NSCLC combined with normal adjacent lung parenchyma. This study was approved by the ethics committee of Zhongnan Hospital of Wuhan University, and informed consent was obtained from each patient undergoing surgery.
Among these 95 NSCLC cases, there were 76 males and 19 females, and their age ranged from 20 to 80 years. The clinical follow-up duration of the patients ranged from 9 to 76 months. Overall survival was calculated from the date of surgery to the date of death or the most recent follow-up (April, 2014). According to the criteria of the Union for International Cancer Control (UICC)/American Joint Committee on Cancer (AJCC), seventh edition, 27, 26, and 42 patients exhibited stage I, stage II, and stage IIIA cancer, respectively. None of the patients received radiotherapy or chemotherapy before curative resection. Systemic adjuvant chemotherapy consisting of a cisplatin-based doublet chemotherapeutic regimen was administered to all patients. Ten patients with stage II cancer and 26 patients with clinical stage IIIA cancer received thoracic postoperative radiotherapy.
Immunohistochemistry
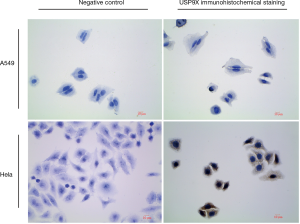
USP9X expression was analyzed immunohistochemically in four micrometer-thick, formalin-fixed, paraffin-embedded sections. The sections were deparaffinized in xylene, rehydrated in ethanol, and treated with a peroxidase-blocking reagent (Dako, Glostrup, Denmark) to abolish endogenous peroxidase activity. The sections were placed in 0.01 mol/L citrate buffer (pH 6.0) for 15 min in a microwave oven and then left at room temperature for 20 min for antigen retrieval. To inhibit non-specific antigen-antibody reactivity in the immunohistochemically stained sections, a protein blocker (Research Genetics, Huntsville, AL, USA) was applied for 5 min, and the sections were washed thoroughly with PBS buffer. Then, the sections were incubated overnight in primary antibodies against USP9X (1:100; rabbit polyclonal antibody, ab19879, Abcam, UK) at 4 degrees centigrade. The specificity of the USP9X antibody (ab19879) was tested in Formalin Fixed and Paraffin Embedded A549 and H1299 cell lines (Figure S1), and the test showed that there was no evidence of cross-immunostaining between USP9X antibody (ab19879) with other proteins. After a PBS wash, the sections were incubated in a goat anti-rabbit secondary antibody (1:200; BA1003, Boster Bio-engineering Limited Company, Wuhan, China) for 20 min at room temperature. After an additional wash step, an avidin-horseradish peroxidase complex was applied for 20 min at room temperature. Finally, the sections were stained with 3,3’-diaminobenzidine (DAB, Sigma-Aldrich, St. Louis, MO, USA), followed by counterstaining with hematoxylin and mounting. All samples served as internal controls, and the sample and control slides were processed simultaneously.
Result evaluation
The results of immunohistochemistry were analyzed by two experienced pathologist (Gui-Fang Yang and Hong Cao) with over 10 years of experience in clinical tumor pathology, and both of them were blinded to the clinicopathological data. The scoring system combined cells staining intensity with cells positive rate. Cells staining intensity was defined as no stain, slight stain, medium stain and strong stain, and correspondingly scored as 0, 1, 2 and 3, respectively. Cells positive rate was defined as grade 0 with none of tumor cells stained positive, grade 1 with positive USP9X immunostaining in 1% to 10% of tumor cells stained positive, grade 2 with 10% to 50% of tumor cells stained positive, grade 3 with 51% to 80% of tumor cells stained positive, and grade 4 with >80% of tumor cells stained positive, and correspondingly scored as 0, 1, 2, 3, 4, respectively. The final score was the sum of cells staining intensity score and cells positive rate score. The sum scores of 0-3 were defined as “negative expression” (−), 4-5 as “weakly positive expression” (+), and 6-7 as “strongly positive expression” (++) (8). All scores were divided into two groups: low expression (scores 0-5) and high expression (scores 6-7) in NSCLC samples.
Statistical analyses
All statistical analyses were performed using SPSS software version 21.0 (SPSS, Inc., Chicago, IL, USA). The association of the clinicopathological characteristics with the USP9X expression status was analyzed using the Pearson’s Chi-squared test or Fisher’s exact test for the categorical variables. To analyze the follow-up data, overall survival was evaluated via Kaplan-Meier analysis and the log-rank test. Univariate and multivariate survival analyses were performed using Cox proportional hazard regression models. A P value <0.05 was considered to be significant.
Results
USP9X is highly expressed in NSCLC
We measured the subcellular localization and the protein levels of USP9X in 95 paraffin-embedded NSCLC tissues and in 32 paraffin-embedded normal lung tissues via immunohistochemical staining (Figure 1). The expression of USP9X was primarily observed in the cytoplasm. High USP9X expression was detected in 42 (44.2%) of the 95 NSCLC samples but only in 2 (6.3%) of the 32 matched normal lung tissue samples. The expression of USP9X was significantly elevated in the NSCLC tissue compared with the normal lung parenchymal tissue (P<0.001, Table 1).
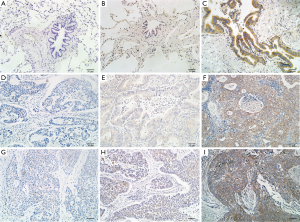

Full table
Association between USP9X expression and the clinicopathological characteristics of the NSCLC patients
The relationship between USP9X expression and the clinicopathological characteristics of the patients with NSCLC is summarized in Table 2. High expression of USP9X was significantly more prevalent among lymph node metastasis-positive cases than among lymph node metastasis-negative cases (63.3% and 23.9%, respectively; P<0.001), and for the NSCLC samples, high expression of USP9X was significantly more frequently observed in advanced clinical stage samples than in early clinical stage samples (64.3% and 28.3%, respectively; P<0.001). Additionally, we observed a trend between USP9X expression and pathologic differentiation, although this trend was not significant (P=0.147). Statistical analysis revealed no significant correlation between USP9X expression and age, gender, smoking status, histological type or tumor size.
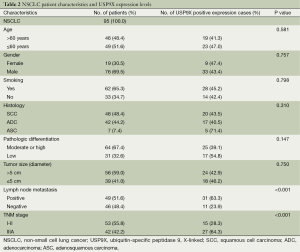
Full table
Survival analysis and prognostic significance of USP9X expression in NSCLC
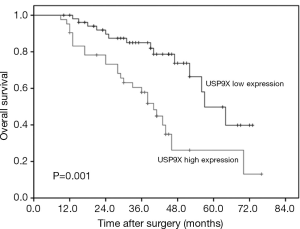
To explore the prognostic value of USP9X expression for NSCLC, we analyzed the association between USP9X expression and patient survival via Kaplan-Meier analysis using the log-rank test. The results showed that the overall survival rate of the patients with high USP9X expression was significantly lower than that of the patients with low USP9X expression (P=0.001) (Figure 2). Furthermore, univariate Cox regression analysis of the NSCLC patients showed that poor overall survival correlated with positive lymph node metastasis (HR =3.421, P=0.001), advanced TNM stage (HR =4.740, P<0.001) and high USP9X expression (HR =3.040, P=0.001). Next, we performed multivariate analysis and found that both clinical stage (HR =5.841, P=0.012) and USP9X expression (HR =2.444, P=0.028) were independent prognostic indicators of overall survival for NSCLC patients (Table 3).
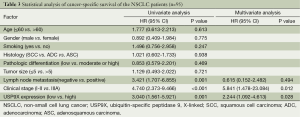
Full table
Discussion
USP9X, a member of the USP family, has been implicated in many biological processes. In embryonic stem cell-derived neural progenitor cells, the USP9X-induced polarization of these neural progenitors results in their radial arrangement, which produces an environment that is conducive of self-renewal (10). In pancreatic acinar cells, USP9X plays a role in pro-survival pathway that affects autophagy (11). In lymphoma cells, USP9X deubiquitinates and stabilizes the pro-survival BCL2 family member MCL1, thereby downregulating apoptosis (9). In other cellular contexts, USP9X promotes cell death. USP9X deubiquitinates and stabilizes Itch (12), an E3 ligase that mediates the degradation of several proteins that support cell survival (13). It has also been reported that the transcriptional activity of SMAD4, a tumor suppressor protein that is inactivated in the majority of pancreatic cancer types (14), can be activated by USP9X (15). These findings indicate that the USP9X is not restricted to the regulation of the polarization of neural stem cells and that this protein is necessary for several processes involved in the modulation of tumor cell death pathways.
USP9X is highly expressed in several types of human cancer, such as esophageal squamous cell carcinoma (8), follicular lymphoma (9) and colon adenocarcinoma (13), compared with the corresponding normal human tissues. However, little is known about USP9X expression in NSCLC patients. In the present study, we found that the USP9X level was higher in the 95 examined NSCLC samples than in the adjacent noncancerous tissues, which was consistent with the previous finding that USP9X is upregulated in NSCLC at the transcriptional level (16). These observations suggest that USP9X may function as an oncogene and may play a role in the pathogenesis of NSCLC. To further elucidate the role of USP9X in the development and progression of lung cancer, we analyzed the expression of USP9X in 95 NSCLC patients and found that elevated USP9X expression was significantly associated with advanced clinical stage (P<0.001) and positive lymph node metastasis (P=0.001). These results suggest that USP9X plays significant roles in NSCLC progression and metastasis. Similarly, in the report by Peng et al. (8), high USP9X expression was positively associated with tumor progression in patients with esophageal squamous cell carcinoma. However, no significant association was detected between USP9X expression and pathologic differentiation of NSCLC (P=0.147), although a clear trend was observed. The uneven distribution of the pathologic differentiation status of these patients may have biased this result.
In the past few years, elevated USP9X expression in tumor cells has been shown to serve as an independent poor prognostic factor for multiple myeloma (9) and esophageal squamous cell carcinoma (8). In this study, we demonstrated that USP9X protein expression in NSCLC was inversely correlated with overall survival, as the patients with higher USP9X protein expression experienced shorter survival duration. According to multivariate analyses, high expression of the USP9X protein was a significant predictor of poor prognosis for NSCLC patients (HR =2.244, P=0.028). These results were consistent with Peng et al.’s (8), report, which suggested that increased expression of USP9X correlated with poor survival among esophageal squamous cell carcinoma patients. Similarly, Schwickart et al. (9), demonstrated that increased USP9X mRNA expression in tumors was significantly associated with poor prognosis among patients with multiple myeloma. However, another study showed that low expression of USP9X protein and mRNA in pancreatic ductal adenocarcinoma was inversely associated with poor prognosis after surgical resection (17). These opposing results may be explained by the tissue-specificity of USP9X in different tumors. As the malignant development of different cell types may be different to some extent, the effect of USP9X on carcinogenesis may be unique in different tissues.
High expression of USP9X may be involved in tumor growth, invasion, and metastasis by regulating certain genes, such as MCL-1, β-catenin, and TGF-β. Recent evidence showed that USP9X expression correlates with that of Mcl-1, a member of the pro-survival BCL-2 family, in human cancer tissues (9). In the same study, the USP9X inhibitor WP1130 was found to promote Mcl-1 degradation and to increase the sensitivity of tumor cells to chemotherapies. Further investigation revealed that USP9X stabilizes Mcl-1 by removing its degradative Lys 48-linked polyubiquitin chains specifically, and that knockdown of USP9X causes a modest decrease in tumor growth. It was reported that USP9X interacts with and stabilizes β-catenin in vivo, presumably via the deubiquitination of β-catenin (18). Interestingly, increased expression of either cytoplasmic or nuclear β-catenin was found to be strongly associated with poor prognosis and was an independent prognostic factor of overall survival in NSCLC (19). Nevertheless, whether the Wnt/β-catenin signaling pathway (20) is activated by the increased expression of USP9X in NSCLC requires further investigation. Alternatively, USP9X might be involved in regulating the TGFβ pathway (21), another signaling circuit that is strongly related to cancer, as demonstrated by the finding that the loss of USP9X abolished multiple TGFβ gene responses (15). In this study, our results provided the first evidence that elevated expression of USP9X correlated with poor prognosis in NSCLC. However, further investigation is required to fully elucidate the exact molecular mechanisms.
Conclusions
In conclusion, the current study demonstrated that the expression of USP9X was significantly increased in NSCLC tissue and that this increase in USP9X expression correlated with poor prognosis among NSCLC patients. Because of the limited sample size of patients in our study, further studies are needed to verify the role of USP9X as a definitive clinical predictor of the prognosis of NSCLC patients.
Acknowledgements
Authors’ contributions: CH Xie designed the overall study. Y Wang and Y Liu contributed by designing and performing the experiments, collecting and analyzing the data, and co-writing the manuscript. H Cao and GF Yang evaluated the immunohistochemical staining of USP9X expression. B Yang, SM Zhang, W Ouyang and CX Yang discussed the manuscript. FX Zhou and YF Zhou revised and edited the manuscript.
Funding: This work was supported by grants from the National Natural Science Foundation of China and the Natural Science Foundation of Hubei Province (grant numbers 81372498 and 2013CFA006, respectively).
Disclosure: The authors declare no conflict of interest.
References
- Siegel R, Ma J, Zou Z, et al. Cancer statistics, 2014. CA Cancer J Clin 2014;64:9-29. [PubMed]
- Rosell R, Karachaliou N. Lung cancer: Maintenance therapy and precision medicine in NSCLC. Nat Rev Clin Oncol 2013;10:549-50. [PubMed]
- Spira A, Ettinger DS. Multidisciplinary management of lung cancer. N Engl J Med 2004;350:379-92. [PubMed]
- Kapuria V, Peterson LF, Fang D, et al. Deubiquitinase inhibition by small-molecule WP1130 triggers aggresome formation and tumor cell apoptosis. Cancer Res 2010;70:9265-76. [PubMed]
- Kerscher O, Felberbaum R, Hochstrasser M. Modification of proteins by ubiquitin and ubiquitin-like proteins. Annu Rev Cell Dev Biol 2006;22:159-80. [PubMed]
- Hussain S, Zhang Y, Galardy PJ. DUBs and cancer: the role of deubiquitinating enzymes as oncogenes, non-oncogenes and tumor suppressors. Cell Cycle 2009;8:1688-97. [PubMed]
- Reyes-Turcu FE, Ventii KH, Wilkinson KD. Regulation and cellular roles of ubiquitin-specific deubiquitinating enzymes. Annu Rev Biochem 2009;78:363-97. [PubMed]
- Peng J, Hu Q, Liu W, et al. USP9X expression correlates with tumor progression and poor prognosis in esophageal squamous cell carcinoma. Diagn Pathol 2013;8:177. [PubMed]
- Schwickart M, Huang X, Lill JR, et al. Deubiquitinase USP9X stabilizes MCL1 and promotes tumour cell survival. Nature 2010;463:103-7. [PubMed]
- Jolly LA, Taylor V, Wood SA. USP9X enhances the polarity and self-renewal of embryonic stem cell-derived neural progenitors. Mol Biol Cell 2009;20:2015-29. [PubMed]
- Grasso D, Ropolo A, Lo Ré A, et al. Zymophagy, a novel selective autophagy pathway mediated by VMP1-USP9x-p62, prevents pancreatic cell death. J Biol Chem 2011;286:8308-24. [PubMed]
- Mouchantaf R, Azakir BA, McPherson PS, et al. The ubiquitin ligase itch is auto-ubiquitylated in vivo and in vitro but is protected from degradation by interacting with the deubiquitylating enzyme FAM/USP9X. J Biol Chem 2006;281:38738-47. [PubMed]
- Bernassola F, Karin M, Ciechanover A, et al. The HECT family of E3 ubiquitin ligases: multiple players in cancer development. Cancer Cell 2008;14:10-21. [PubMed]
- Hahn SA, Schutte M, Hoque AT, et al. DPC4, a candidate tumor suppressor gene at human chromosome 18q21.1. Science 1996;271:350-3. [PubMed]
- Dupont S, Mamidi A, Cordenonsi M, et al. FAM/USP9x, a deubiquitinating enzyme essential for TGFbeta signaling, controls Smad4 monoubiquitination. Cell 2009;136:123-35. [PubMed]
- Luise C, Capra M, Donzelli M, et al. An atlas of altered expression of deubiquitinating enzymes in human cancer. PLoS One 2011;6:e15891. [PubMed]
- Pérez-Mancera PA, Rust AG, van der Weyden L, et al. The deubiquitinase USP9X suppresses pancreatic ductal adenocarcinoma. Nature 2012;486:266-70. [PubMed]
- Taya S, Yamamoto T, Kanai-Azuma M, et al. The deubiquitinating enzyme Fam interacts with and stabilizes beta-catenin. Genes Cells 1999;4:757-67. [PubMed]
- Li XQ, Yang XL, Zhang G, et al. Nuclear β-catenin accumulation is associated with increased expression of Nanog protein and predicts poor prognosis of non-small cell lung cancer. J Transl Med 2013;11:114. [PubMed]
- Stewart DJ. Wnt signaling pathway in non-small cell lung cancer. J Natl Cancer Inst 2014;106:djt356. [PubMed]
- Vizán P. Response to comment on "Controlling long-term signaling: receptor dynamics determine attenuation and refractory behavior of the TGF-β pathway"-Smad2/3 activity does not predict the dynamics of transcription. Sci Signal 2014;7:lc2. [PubMed]


