How well does pathologic stage predict survival for esophageal adenocarcinoma after neoadjuvant therapy?
Introduction
The value of a cancer staging system lies in its ability to predict survival. The 7th edition of the American Joint Committee on Cancer (AJCC7) Cancer Staging Manual for esophageal cancer was guided by survival data, using random forest methodology (1). The staging was constructed based on characteristics from 4,627 patients from 13 institutions in five countries who underwent esophagectomy. The majority of the patients (n=2,775, 60%) had adenocarcinoma (2). The staging was based on patients who were treated with surgery only, without induction or adjuvant therapy. However, for patients who present with locally advanced disease, neoadjuvant chemoradiation has become the standard of care (3). The prognostic value of the pathologic stage is unknown for these patients who have received preoperative radiation treatment. It is unclear whether the prognosis of a pathologic T1N0 tumor after radiation is the same as a T1N0 tumor without radiation.
Rizk and colleagues found that the previous edition of the AJCC staging system was not a good predictor of survival in patients receiving multimodality therapy for esophageal adenocarcinoma, noting that pathologic T stage was not closely associated with survival (4). Another study examining clinical staging of esophageal squamous cell cancer (SCC) treated with definitive chemoradiation found that the 7th edition staging system did not accurately stratify survival for patients with advanced stages, indicating that the staging may be less accurate for patients receiving chemoradiation as opposed to surgery alone (5).
Many patients experience pathologic downstaging after chemoradiation, although some have no response (6). The prognostic importance of the pathologic stage after receiving neoadjuvant chemoradiation is unknown (7). Therefore, we explored the prognostic accuracy of the 7th edition AJCC staging system for esophageal cancer in a large, population-based cancer registry, which includes patients who received multimodality therapy.
Methods
Patient selection
Patient data contained within the Surveillance Epidemiology and End Results (SEER) dataset was analyzed. The SEER catchment area covers approximately 28% of the US and the dataset contains both clinicopathologic information as well as outcome data (overall survival and disease specific survival). Tumor location, grade, and histology were coded according to the International Classification of Diseases for Oncology (ICD-O), version ICDO-3. Tumor stage was coded according to AJCC TNM staging system, 7th edition. Given the lack of patient specific identifying information, IRB approval was waived.
From 1988 to 2009, a total of 52,785 patients with esophageal cancer were identified. Our analysis was restricted to patients with the following characteristics: age ≥18, disease stage groups I-III, adenocarcinoma histology (ICD-O codes 8140-8151, 8154-8231, 8243-8245 or 8250-8576), had undergone surgical resection, did not receive adjuvant radiation, and who were definitively pathologically staged. To ensure that patients were in fact pathologically staged, we excluded anyone who was stage N1 or above but had 0 examined nodes, 0 positive nodes or an unknown positive node status. In addition, anyone who had a SEER extension or node classification code defined as “does not meet criteria for AJCC pathologic staging” was excluded. Lastly, anyone diagnosed from July 1 to December 31, 2005 in a Hurricane Katrina impacted area was excluded (n=6). In total, 4,529 patients were included in the analysis.
Statistical analysis
The primary purpose of this study was to explore the prognostic accuracy of the 7th edition AJCC staging system as it pertains to esophageal cancer patients, specifically those who underwent resection with or without neoadjuvant radiation. Patients were compared across neoadjuvant radiation groups (none vs. any) using a Student’s t-test for continuous variables and a chi-squared test for categorical variables. Missing and unknown values were excluded in the variable specific analyses. Kaplan-Meier survival curves were used to calculate median, 2- and 5-year survival rates, with the log-rank test used to determine statistical differences across groups. Survival was calculated from the date of diagnosis until the date of death if the patient had died. If the patient was alive at last contact, the patient was censored at the date of last contact. For all statistical analyses, SAS software was used. P values cited herein are two sided with those values less than 0.05 considered statistically significant.
Results
From 1988-2009, 4,529 patients in the database met inclusion criteria; 1,243 patients received preoperative radiation and 3,286 did not (Table 1). Patients receiving preoperative radiation were younger (P<0.0001) and had higher grade tumors (P<0.001). Less than a third (380/1,243) of the patients in the radiation group had pathologic T1 tumors compared to nearly half (1,534/3,286) in the other group. A slightly lower percentage of patients in the radiation group had N0 disease (58% vs. 63%).
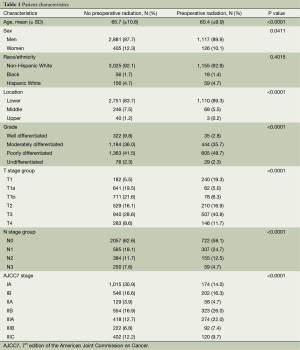
Full table
For those patients who underwent surgery without preoperative radiation, the 7th edition AJCC staging system for adenocarcinoma was an excellent predictor of survival (log-rank chi-squared =1,164.7). There was good separation of all stages and stage sub-groupings (Figure 1A). For patients who underwent surgery after radiation, the staging system was not as accurate for prediction of survival and there was less distinction among stage subgroups (P<0.001, log-rank chi-squared =81.8). Of note, median survival was greater for patients with stage IIIC than patients with IIIB disease (Figure 1B).

When comparing the stage specific survival for patients who received preoperative radiation and those who did not, we found that survival for patients with pathologic stage I was better for those who did not receive radiation, whereas for stages II and III, survival was significantly improved for patients who had radiation (Table 2).
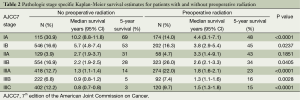
Full table
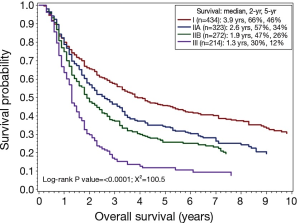
Recognizing the lack of distinction among stage subgroups, we investigated whether an alternative, simpler stage grouping could better stratify patients who had preoperative radiation (Table 3). We selected the stage groupings to reflect the finding that patients with N2 and N3 disease had similarly poor prognosis regardless of T stage. This alternative staging system had an improved chi-squared value of 100.5 (Figure 2). The alternative staging system worked well to stratify patients in the non-radiated group as well (log-rank chi-squared =1,103).
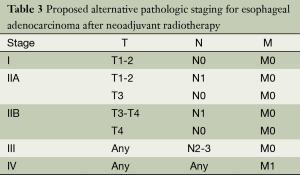
Full table
Conclusions
The goal of a staging system for cancer is to group patients with similar prognosis. Rice and colleagues define the attributes of a good staging system as: decreasing patient survival with increasing stage group (monotonicity), difference in survival between groups (distinctiveness), and similar survival within a group (homogeneity) (1). Our analysis of the SEER dataset reveals that the current, 7th edition AJCC staging system for esophageal adenocarcinoma is an excellent staging system for patients who did not receive preoperative radiation, possessing all the aforementioned attributes. This study is the largest validation of the staging system. Indeed, the control group included more patients with adenocarcinoma than the original WECC database on which the staging was based (3,286 vs. 2,775).
For those patients who received preoperative radiation therapy, the staging system was far less predictive of survival, with a significantly lower chi-square value. In particular, there was less distinctiveness among subgroups. Part of this was a reflection of a narrower distribution in survival across all groups. The 5-year survival for stage IA was only 48% in the radiotherapy group compared to 63% in the non-radiated group. Conversely, the 5-year survival for stage IIIC patients was 15% in the radiated group compared to 4% in the non-radiated group. Thus, for patients receiving pre-operative radiation, there was only a 33% absolute survival difference at 5 years between the earliest and most advanced stages. This is compared to a 59% absolute survival difference in the non-radiated group.
A stage specific comparison of the non-radiated and radiated groups shows that survival for pathologic stage I patients was worse in the radiated group. Presumably, there was some stage migration and these patients were downstaged with pre-operative therapy, since neoadjuvant therapy is not generally given for patients with stage I disease. Alternatively, these patients may have been clinically overstaged, but this is relatively unlikely because the rate of clinical overstaging from stage I to stage II or higher is low (8). Regardless, the data does indicate that pathologic stage I after chemoradiation does not confer the same prognosis as a pure pathologic stage I tumor.
In contrast, radiated patients with pathologic stage II and III disease have improved survival compared to non-radiated patients. Numerous randomized clinical trials have now shown that patients with locally advanced disease have improved survival with multimodality therapy compared to surgery alone (9-12). Patients with a pathologic complete response had the most benefit from neoadjuvant therapy. However, the data from our study indicate that the improvement in survival is not simply due to downstaging. At the more advanced pathologic stages (II and III), there appears to be improved survival for patients who received neoadjuvant radiation.
The relatively narrow distribution in survival among all the patients who receive preoperative radiation may have widened if the study had included neoadjuvant patients who had pathologic complete response. However, currently, there is no pathologic stage for these patients. Nor is there a pathologic stage for patients who are ypT0N1. Our observation that there was a loss of distinctiveness among stage subgroups led us to explore the accuracy of a different, simpler staging system for patients who received neoadjuvant radiation. Having a separate staging system for these patients would undoubtedly complicate things for clinicians, however the alternative staging system does help illustrate the decreased precision and decreased predictive ability of the AJCC staging system for patients receiving multimodality therapy. This is an important consideration as multimodality therapy becomes the standard of care for locally advanced esophageal cancer.
Our conclusions are limited by the information available in the dataset. The SEER database does not include information about neoadjuvant chemotherapy. Therefore some of the patients in the non-radiated group likely received neoadjuvant chemotherapy. In addition, it is unclear how many of the patients in the neoadjuvant radiation group received chemoradiation vs. radiation alone. Because neoadjuvant radiation alone is not a common practice, this is unlikely to be a significant number. Finally, the database only includes information about whether the radiation was given before surgery, not the exact timing or intent of the radiation. Thus, we do not know how many of the patients in the preoperative radiation group actually had surgery as salvage treatment, as opposed to planned surgery after neoadjuvant therapy.
Regardless, the main conclusions of our study remain valid. After patients receive radiation, the final pathologic stage has different prognostic significance compared to patients who have surgery alone. Patients with pathologic stage II or III after radiation and surgery have better survival than patients with pathologic stage II or III after surgery alone. As more patients receive multimodality therapy for esophageal cancer, it will be important to develop better ways to predict prognosis for these patients.
Acknowledgements
Authors’ contributions: JY Kim designed the overall study and wrote the paper. RA Nelson collected and analyzed the data. J Kim and D Raz discussed and edited the paper.
Disclosure: The authors declare no conflict of interest.
References
- Rice TW, Rusch VW, Ishwaran H, et al. Cancer of the esophagus and esophagogastric junction: data-driven staging for the seventh edition of the American Joint Committee on Cancer/International Union Against Cancer Cancer Staging Manuals. Cancer 2010;116:3763-73.
- Rice TW, Blackstone EH, Rusch VW. 7th edition of the AJCC Cancer Staging Manual: esophagus and esophagogastric junction. Ann Surg Oncol 2010;17:1721-4.
- Enestvedt CK, Perry KA, Kim C, et al. Trends in the management of esophageal carcinoma based on provider volume: treatment practices of 618 esophageal surgeons. Dis Esophagus 2010;23:136-44. [PubMed]
- Rizk NP, Venkatraman E, Bains MS, et al. American Joint Committee on Cancer staging system does not accurately predict survival in patients receiving multimodality therapy for esophageal adenocarcinoma. J Clin Oncol 2007;25:507-12. [PubMed]
- Nomura M, Shitara K, Kodaira T, et al. Prognostic impact of the 6th and 7th American Joint Committee on Cancer TNM staging systems on esophageal cancer patients treated with chemoradiotherapy. Int J Radiat Oncol Biol Phys 2012;82:946-52. [PubMed]
- Ajani JA, Correa AM, Hofstetter WL, et al. Clinical parameters model for predicting pathologic complete response following preoperative chemoradiation in patients with esophageal cancer. Ann Oncol 2012;23:2638-42. [PubMed]
- Swisher SG, Hofstetter W, Komaki R, et al. Improved long-term outcome with chemoradiotherapy strategies in esophageal cancer. Ann Thorac Surg 2010;90:892-8; discussion 898-9. [PubMed]
- Stiles BM, Mirza F, Coppolino A, et al. Clinical T2-T3N0M0 esophageal cancer: the risk of node positive disease. Ann Thorac Surg 2011;92:491-6; discussion 496-8. [PubMed]
- Gebski V, Burmeister B, Smithers BM, et al. Survival benefits from neoadjuvant chemoradiotherapy or chemotherapy in oesophageal carcinoma: a meta-analysis. Lancet Oncol 2007;8:226-34. [PubMed]
- Tepper J, Krasna MJ, Niedzwiecki D, et al. Phase III trial of trimodality therapy with cisplatin, fluorouracil, radiotherapy, and surgery compared with surgery alone for esophageal cancer: CALGB 9781. J Clin Oncol 2008;26:1086-92. [PubMed]
- Walsh TN, Noonan N, Hollywood D, et al. A comparison of multimodal therapy and surgery for esophageal adenocarcinoma. N Engl J Med 1996;335:462-7. [PubMed]
- van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 2012;366:2074-84. [PubMed]


