Impact of daily bathing with chlorhexidine gluconate on ventilator associated pneumonia in intensive care units: a meta-analysis
Introduction
Ventilator associated pneumonia (VAP) represents one of the most important nosocomial infections in critical ill patients with increased longer duration of mechanical ventilation, greater number of intensive care unit (ICU) days, hospital costs, and higher mortality (1,2). Pooled VAP density in adult ICUs in developing countries was 22.9 per 1,000 ventilator-days, at least four times as high as densities reported from the developed countries (3).
“Bundles” are a set of processes of care have been taken to prevent morbidity of VAP (4), such as semi-recumbent body position (5), hand hygiene, daily sedation vacations (6,7), oral care with chlorhexidine gluconate (CHG) (8,9) and so on. CHG is a classic broad-spectrum antimicrobial activity with a good safety profile against gram-positive and gram-negative bacteria and safety profile (10,11). Recently, there has been a renewed interest in this antiseptic as a crucially complementary measure to prevent acquired central line associated bloodstream infection (CLABSI), surgical site infection (SSI) and antimicrobial-resistant bacteria (12,13) among critical ill patients, suggesting its robust effects of reducing global but not specific infection rates. Interestingly, Martínez-Reséndez et al. revealed the potential role in preventing VAP (14). However, the results remain conflicting rather than conclusive (12,15). Therefore, this meta-analysis was performed to investigate the association between daily bathing with CHG and incidence of VAP.
Materials and methods
The whole procedures of this meta-analysis adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement guidelines (16).
Search strategy
An electronic search engine (PubMed), Embase and the Cochrane Central Register database were searched separately up to June 1 2014, for all eligible studies by two different reviewers (W Chen and H Li). An electronic search was performed using the following terms: “chlorhexidine”, “Ventilator associated pneumonia”, “VAP”, “chlorhexidine bath*”, “chlorhexidine washcloth*”. Additional studies were identified by a hand search of references of original studies or review articles on this topic. No language restrictions were imposed. The three independent investigators (W Chen, Q Cao and W Zhang) reached consistency on all data sets for this manuscript.
Eligibility criteria
All clinical randomized controlled trials (RCTs), quasi-experimental studies that investigated the efficacy of daily using CHG bathing to prevent the morbidity of VAP among critical ill adult patients in ICU settings were eligible in this study. Studies which have been published in full-articles, and reported the number of intervention and control were included in the latter analysis. CHG bathing which was not applied as the primary part of intervention was excluded (17,18).
Data extraction
Both authors (S Li and H Li) extracted the data independently using a data extraction form. Disagreement was settled by consensus between all authors. Information on study design, setting, study population, nature of interventions, co-interventions was collected.
Quality assessment
A key feature of the Grades of Recommendations Assessment Development and Evaluation (GRADE) method developed by the Cochrane review group was used to assess the quality rather than individual study (19,20). Four categories of quality ratings in GRADE—“high”, “moderate”, “low” and “very low” on the representativeness of risks of bias, inconsistency, indirectness, imprecision and publication bias (Table 1) (23). Two authors (W Chen and S Li) assessed the quality of evidence independently following GRADE guidance (19). Disagreement between authors was resolved by discussion and finally judged by the third reviewer (W Zhang).
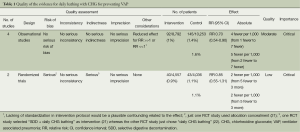
Full table
Statistical analysis
If the between-study heterogeneity was found, a random-effect model was conducted. If I2 was ≤50%, a fixed effects model was used to calculate a pooled estimate of effect; if the I2 statistic was >50%, a random effect model was used (15). Publication bias was evaluated by the linear regression asymmetry test by Egger et al. (24). All data were analyzed in Review Manager (v.5.1.6; Oxford, England) and STATA11.0 (Stata-Corp, College Station, Tex).
Results
Figure 1 summarizes the diagram of selection process. From an initial 180 potentially relevant articles, we included six in our final analysis with two RCTs and four quasi-experimental studies (Figure 1).
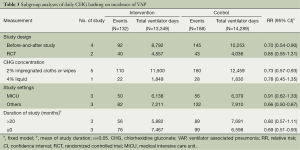
Table 1 shows the methodological quality of included trials following the GRADE method (Table 1). All of these available studies were conducted in ICU settings (intervention/control: 13,349 ventilator days/14,289 ventilator days) (14,23-27) (Table 2).
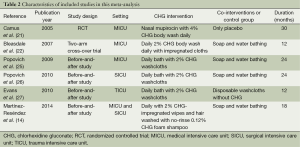
Full table
We found that daily bathing with CHG would decreased 27% risk of VAP in ICU settings [relative risk (RR): 0.73, 95% confidence interval (CI): 0.59-0.92, I2=0%] (Figure 2). Figure 2 and Table 3 summarize the subgroup analysis in this review. We found that daily bathing with CHG would lower incidence risk of VAP especially in subgroup of 2% CHG impregnated cloths or wipes (14,22,25-27) (RR: 0.73, 95% CI: 0.57-0.93) and before-and-after studies (14,25-27) (RR: 0.70, 95% CI: 0.54-0.90) (Table 3). Meanwhile, daily bathing with CHG may also decrease the incidence risk of VAP for two RCT studies, although no significant association was found (21,22) (RR: 0.85, 95% CI: 0.55-1.31) and longer study duration (>20 months) (21,25,26) (RR: 0.80, 95% CI: 0.57-1.11) (Table 3).
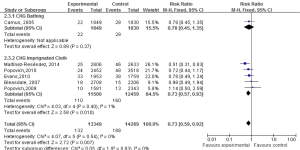
Full table
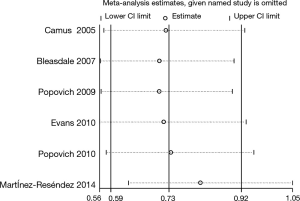
A sensitivity analysis by omitting individual study was performed in this meta-analysis to assess the impact of each individual study on the pooled RRs. We found the pooled RRs would not be significantly affected by omitting one multiple-center, placebo-controlled, randomized, double-blind study (21) (RR: 0.73, 95% CI: 0.57-0.93). However, one quasi-experimental study played an important role in the pooled RRs (Figure 3) (remained RR: 0.81, 95% CI: 0.63-1.05) (14).
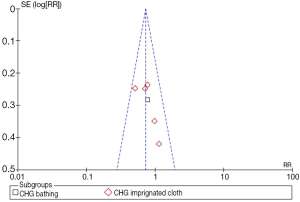
The funnel plot is used to investigate the publication bias for VAP. In our meta-analysis the funnel is asymmetric, suggesting existed publication bias (Figure 4).
Discussion
The impact of daily bathing with CHG to prevent health-care associated infection (HAI), such as CLABSI (17), SSI (15), multi-drug resistant organisms (MDROs) acquisition (12) and so on, has been well investigated in some clinical trials. However, there were limited studies to explore the association between daily bathing with CHG and incidence risk of VAP among critical ill patients undergoing mechanical ventilation. In this meta-analysis, we reviewed the published epidemiological reports on the role of daily CHG bathing which would low the incidence risk of VAP (RR: 0.73, 95% CI: 0.59-0.92). Meanwhile, we found that daily bathing with 2% CHG impregnated cloth or wipes would also decrease VAP risk among critical ill patients (RR: 0.73, 95% CI: 0.57-0.93). Our findings suggest that daily bathing with CHG would reduce the risk of VAP in ICU settings.
The precise mechanism to explain the association between daily bathing with CHG and VAP reduction remains unknown. In 2005, Vernon et al. (28) performed a prospective single-arm clinical trial in a medical ICU. A total of 1,787 patients were bathed or cleansed and assessed for acquisition of Vancomycin-Resistant Enterococcus (VRE). They found that cleansing patients with chlorhexidine saturated cloths significantly lowed VRE contamination of patients’ skin, the environment (RR: 0.3, 95% CI: 0.2-0.5) and health care workers’ hands (RR: 0.6, 95% CI: 0.4-0.8) and to decrease patient acquisition of VRE (RR: 0.3, 95% CI: 0.2-0.5) (28), suggesting the great role of daily CHG bathing in decreasing the “colonization pressure” which was a momentous risk factor for HAIs (29), and in reducing the risk of subsequent infection from manipulation of devices associated with the patient (14), interrupting the cross-infection in ICU settings. Above evidences may account for the potential role of daily CHG bathing in preventing the morbidity of VAP to some extent, which was consistent with the findings of our meta-analysis.
However, some important concerns merit more consideration and caution. First of all, the overall effect was more significant in before-and-after studies compared with the pooled effect from two RCT studies (Table 3). Moreover, one well designed RCT did significantly affect the pooled RRs (21), though the pooled RR would be affected by another quasi-experimental study (14) in sensitivity analysis. Our findings suggested that further well-designed studies should be performed to clarify the benefit of daily bathing with CHG for preventing VAP. In this review, GRADE method was also applied to assess the quality of this study. The benefit between the daily bathing with CHG and acquired VAP was found in observational studies and no significant heterogeneity was tested, the quality of evidence was rated “moderate” according to the GRADE system (Table 1). Two eligible RCT studies (21,22) were pooled in this meta-analysis, and we found that daily bathing with CHG might be associated with lower risk of VAP (RR: 0.85, 95% CI: 0.55-1.31), though the test for overall effect was not significant (Z=0.73, P=0.46). Recently, although daily bathing with CHG shown some benefits in preventing nosocomial infections regardless of CHG bathing is done using CHG impregnated cloths or a liquid preparation, the standard intervention protocol was still not established. In additional, conceal allocation was not available in one RCT (22). Eventually, we degraded the quality of the evidence and rated “low” according to the GRADE system (Table 1). Nevertheless, the crucial impact of the daily bathing with CHG in preventing VAP should not be neglected by infection preventionists (IPs) or health-care workers (HCWs).
In conclusion, existing data—even if mainly obtained from quasi-experimental studies—support the practice of daily bathing with CHG for reducing VAP for critical ill patients. Additional well-designed large studies were required for the validation of this association.
Acknowledgements
Funding: This study was supported by grants from Jiangsu Province Projects of Preventive Medicine Research (Y2013044) and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD) and JX10231801.
Disclosure: The authors declare no conflict of interest.
References
- Lambert ML, Suetens C, Savey A, et al. Clinical outcomes of health-care-associated infections and antimicrobial resistance in patients admitted to European intensive-care units: a cohort study. Lancet Infect Dis 2011;11:30-8. [PubMed]
- Vincent JL, Rello J, Marshall J, et al. International study of the prevalence and outcomes of infection in intensive care units. JAMA 2009;302:2323-9. [PubMed]
- Allegranzi B, Bagheri Nejad S, Combescure C, et al. Burden of endemic health-care-associated infection in developing countries: systematic review and meta-analysis. Lancet 2011;377:228-41. [PubMed]
- Nolan T, Berwick DM. All-or-none measurement raises the bar on performance. JAMA 2006;295:1168-70. [PubMed]
- IHI. Implement the IHI Ventilator Bundle. Available online: http://www.ihi.org/knowledge/Pages/Changes/ImplementtheVentilatorBundle.aspx
- Kress JP, Pohlman AS, O'Connor MF, et al. Daily interruption of sedative infusions in critically ill patients undergoing mechanical ventilation. N Engl J Med 2000;342:1471-7. [PubMed]
- Girard TD, Kress JP, Fuchs BD, et al. Efficacy and safety of a paired sedation and ventilator weaning protocol for mechanically ventilated patients in intensive care (Awakening and Breathing Controlled trial): a randomised controlled trial. Lancet 2008;371:126-34. [PubMed]
- Climo MW, Yokoe DS, Warren DK, et al. Effect of daily chlorhexidine bathing on hospital-acquired infection. N Engl J Med 2013;368:533-42. [PubMed]
- Chan EY, Ruest A, Meade MO, et al. Oral decontamination for prevention of pneumonia in mechanically ventilated adults: systematic review and meta-analysis. BMJ 2007;334:889. [PubMed]
- Milstone AM, Passaretti CL, Perl TM. Chlorhexidine: expanding the armamentarium for infection control and prevention. Clin Infect Dis 2008;46:274-81. [PubMed]
- Rosenberg A, Alatary SD, Peterson AF. Safety and efficacy of the antiseptic chlorhexidine gluconate. Surg Gynecol Obstet 1976;143:789-92. [PubMed]
- Derde LP, Dautzenberg MJ, Bonten MJ. Chlorhexidine body washing to control antimicrobial-resistant bacteria in intensive care units: a systematic review. Intensive Care Med 2012;38:931-9. [PubMed]
- Chen W, Li S, Li L, et al. Effects of daily bathing with chlorhexidine and acquired infection of methicillin-resistant Staphylococcus aureus and vancomycin-resistant Enterococcus: a meta-analysis. J Thorac Dis 2013;5:518-24. [PubMed]
- Martínez-Reséndez MF, Garza-González E, Mendoza-Olazaran S, et al. Impact of daily chlorhexidine baths and hand hygiene compliance on nosocomial infection rates in critically ill patients. Am J Infect Control 2014;42:713-7. [PubMed]
- Karki S, Cheng AC. Impact of non-rinse skin cleansing with chlorhexidine gluconate on prevention of healthcare-associated infections and colonization with multi-resistant organisms: a systematic review. J Hosp Infect 2012;82:71-84. [PubMed]
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097. [PubMed]
- O'Horo JC, Silva GL, Munoz-Price LS, et al. The efficacy of daily bathing with chlorhexidine for reducing healthcare-associated bloodstream infections: a meta-analysis. Infect Control Hosp Epidemiol 2012;33:257-67. [PubMed]
- Fraser TG, Fatica C, Scarpelli M, et al. Decrease in Staphylococcus aureus colonization and hospital-acquired infection in a medical intensive care unit after institution of an active surveillance and decolonization program. Infect Control Hosp Epidemiol 2010;31:779-83. [PubMed]
- Balshem H, Helfand M, Schünemann HJ, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol 2011;64:401-6. [PubMed]
- Atkins D, Best D, Briss PA, et al. Grading quality of evidence and strength of recommendations. BMJ 2004;328:1490. [PubMed]
- Camus C, Bellissant E, Sebille V, et al. Prevention of acquired infections in intubated patients with the combination of two decontamination regimens. Crit Care Med 2005;33:307-14. [PubMed]
- Bleasdale SC, Trick WE, Gonzalez IM, et al. Effectiveness of chlorhexidine bathing to reduce catheter-associated bloodstream infections in medical intensive care unit patients. Arch Intern Med 2007;167:2073-9. [PubMed]
- Warren-Gash C, Fragaszy E, Hayward AC. Hand hygiene to reduce community transmission of influenza and acute respiratory tract infection: a systematic review. Influenza Other Respir Viruses 2013;7:738-49. [PubMed]
- Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629-34. [PubMed]
- Popovich KJ, Hota B, Hayes R, et al. Effectiveness of routine patient cleansing with chlorhexidine gluconate for infection prevention in the medical intensive care unit. Infect Control Hosp Epidemiol 2009;30:959-63. [PubMed]
- Popovich KJ, Hota B, Hayes R, et al. Daily skin cleansing with chlorhexidine did not reduce the rate of central-line associated bloodstream infection in a surgical intensive care unit. Intensive Care Med 2010;36:854-8. [PubMed]
- Evans HL, Dellit TH, Chan J, et al. Effect of chlorhexidine whole-body bathing on hospital-acquired infections among trauma patients. Arch Surg 2010;145:240-6. [PubMed]
- Vernon MO, Hayden MK, Trick WE, et al. Chlorhexidine gluconate to cleanse patients in a medical intensive care unit: the effectiveness of source control to reduce the bioburden of vancomycin-resistant enterococci. Arch Intern Med 2006;166:306-12. [PubMed]
- Bonten MJ, Slaughter S, Ambergen AW, et al. The role of "colonization pressure" in the spread of vancomycin-resistant enterococci: an important infection control variable. Arch Intern Med 1998;158:1127-32. [PubMed]


