Intrapleural tissue plasminogen activator and deoxyribonuclease therapy for pleural infection
Introduction
Pleural infection is increasing worldwide with many reports showing significant rises in the incidence over the last few decades (1-6). In the United States alone these infections are responsible for 90,000 hospital admissions a year (2) and contribute to significant mortality, morbidity and healthcare costs. The mortality in adult patients approaches 20% (3,7) and is skewed towards the elderly (5,8) and those with co-morbidities (6). Up to 50% of patients are referred to surgical intervention (3,9), often with considerable length of hospital stay and costs (7,9).
Treatment of empyema with open thoracic drainage was described by Hippocrates (10). Evacuation of the infected pleural fluid by chest tube, in conjunction with antibiotic therapy, has since been the cornerstone of management (1). However, infected pleural fluid can be difficult to drain due to the presence of fibrous septations and the high fluid viscosity. Surgical intervention to physically breakdown the septations is often needed, especially when drainage is inadequate and sepsis persists. Surgery has recognized morbidity (11) and is often restricted to those with low peri-operative risk (3). Research efforts have focused on exploring effective intrapleural therapies as alternatives to surgery.
The optimal intrapleural therapy should maximize removal of the infected pleural fluid, achieve control of sepsis and consequently negate the need for surgery. Ideally the intrapleural therapy should be practical, safe and cost effective. With this intent, various fibrinolytic agents have been studied since the 1940s with variable outcomes (7,9,12-16).
The intrapleural use of tissue plasminogen activator (tPA) and human recombinant deoxyribonuclease (DNase) in the management of pleural infection has attracted considerable interest. Recent literature supports this combination therapy as an exciting advance that can change the approach to pleural infection management (9,17). We review the pharmacology and biological mechanisms of these drugs as well as the clinical evidence supporting their use.
Historical perspective
Fibrinolytic therapy
Intrapleural fibrinolytic therapy was first described in the late 1940’s. Tillet et al. (18) used a partially purified streptococcal concentrate containing streptokinase and streptococcal DNase intrapleurally in patients with fibrinous pleurisy and empyema. They concluded that the reduction in fibrinogen levels and increase in markers of proteolysis following administration was a representation of fibrin breakdown by streptokinase within the pleural space. A reduction in purulent pleural exudate viscosity was attributed to the activities of streptococcal DNase.
Resurgence of streptokinase use occurred in the late 1980s with successful case series reported in both adults and children (19-21). Strange et al. showed in an animal model of empyema greater production of pleural fluid and less pleural adhesions with intrapleural streptokinase compared with saline, though the amount of pleural thickening remained the same (22).
Urokinase, a thrombolytic agent derived from human neonatal kidney cells was also investigated. Case series showed that intrapleural urokinase therapy improved pleural fluid drainage and reduced need for further surgical intervention (23,24). Bouros et al. conducted the only head-to-head randomised, double-blinded trial of streptokinase (250,000 IU) vs. urokinase (100,000 IU) and found that both drugs increased pleural fluid drainage (12). Another randomised, double-blinded, placebo-controlled study of 31 patients showed that urokinase enhanced pleural fluid drainage, and reduced the need of surgery (14). Diacon et al. showed, in a randomized study, that intrapleural streptokinase therapy reduced surgical referrals, over placebo, in one of the time points tested (15)—a result that could be interpreted as a chance event given the small cohort of 44 patients (25).
The limitations of the small aforementioned studies, and the ongoing debate on the role of fibrinolytics brought about the Multicenter Intrapleural Sepsis Trial (MIST)-1, the largest randomized placebo-controlled trial of fibrinolytic therapy in pleural infection (7). The patients (n=427) were randomised to receive intrapleural instillations of streptokinase (250,000 IU) or placebo twice daily for three days. Streptokinase offered no benefits in rate of surgery, survival, radiographic outcomes, or length of hospital stay; but induced more adverse events (chest pain, fever, or allergy).
DNase therapy
Deoxyribonucleoprotein content exerts a major influence in increasing the viscosity of pus in the pleural space. Simpson et al. proved the principle that addition of DNase allowed significantly faster passage of pus (collected from nine patients with empyema or other abscesses) as measured using a home-made device (26). Light et al. then confirmed the effectiveness of combination therapy of DNase and streptokinase in reducing pus viscosity compared with streptokinase alone, urokinase or saline (27). Case reports of successful use of DNase began to emerge in selected patients (28).
Further animal studies also confirmed the synergistic benefits of combination therapy of tPA (alteplase) and DNase. Zhu et al. showed that the combination treatment provided larger amounts of pleural fluid and improvement in empyema scores across all other comparisons with tPA, DNase, or saline (29). These promising data led to the examination of this combination therapy in humans in the recent MIST-2 study (9).
Pharmacology of tPA and DNase
The individual mechanisms of action of tPA as a thrombolytic/fibrinolytic drug and DNase as a mucolytic agent are well described. Limited data exist on their action and pharmacokinetics when administered intrapleurally and in the setting of pleural infection.
Recombinant tPA
Recombinant tPA, or alteplase, is a recognized systemic treatment for myocardial infarction, pulmonary embolism and thromboembolic stroke. Similar to other fibrinolytics, tPA converts plasminogen to the active protease plasmin, which degrades fibrin into soluble products. A unique characteristic of tPA is that it is fibrin-selective and preferentially activates plasminogen at the surface of a clot.
Multi-loculated effusions are a feature of complicated parapneumonic effusions and empyema. An imbalance between activators and inhibitors of the fibrinolytic system creates a pro-fibrotic state (30-34). Elevated levels of plasminogen activator inhibitor (PAI)-1 are correlated with those of inflammatory mediators, including tumor necrosis factor-α, interleukin-8, and transforming growth factor-β within the pleural space (33). These cytokines are elevated in pleural infection and capable of stimulating PAI-1 release from mesothelial cells (34). Reduced levels of endogenous pleural tPA in this setting are also described (31). The resultant pro-fibrotic state leads to fibrin deposition and loculations within the infected pleural space.
Intrapleural fibrinolytics lyse pleural adhesions by activation of plasmin, aiding drainage of the effusion by breaking down fibrinous septations (35). Given early in the fibrinopurulent phase they may also prevent ongoing fibrin deposition and reduce the severity of loculations by counteracting the profibrotic milieu found within the infected pleural space.
The theoretical advantage of combination therapy incorporating a fibrinolytic agent and DNase, to lyse adhesions and thin pus respectively, is attractive. However the choice of fibrinolytic agent in this combination therapy is debated. To date, only tPA has been formally studied together with DNase, although isolated reports have described combination therapy of streptokinase and DNase (18,28).
Repeated systemic administration of streptokinase has been linked with a higher incidence of allergic reaction and formation of anti-streptokinase antibody which may reduce its therapeutic efficacy (7,36). Antibodies to streptokinase have been reported following intrapleural streptokinase instillations but may also develop following infections with Streptococcal species (37). The influence of these antibodies on clinical outcomes remains unclear. Nonetheless non-antigenic fibrinolytic agents, e.g., tPA and urokinase, may offer potential advantage over streptokinase. In one case report, two courses of intrapleural tPA with DNase were administered sequentially to separate pleural fluid collections in the same patient with good clinical outcome and no adverse effects (38).
Bleeding is often a concern of intrapleural administration of fibrinolytics. At clinical doses, tPA can activate circulating plasminogen resulting in a systemic lytic state and bleeding risks (39). Severe bleeding occurred in 1.8% of patients following intravenous administration of streptokinase or tPA for myocardial infarction, whereas moderate bleeding requiring transfusions was reported in 11.8% (40). Given intravenously, tPA is rapidly metabolised in the liver and undergoes biphasic elimination with an initial half-life of 3-5 minutes, followed by an elimination half-life of 27-46 minutes. Both streptokinase and urokinase also demonstrate rapid systemic elimination.
The degree of systemic absorption of tPA from the pleural space is unknown, but is likely to be low. This, coupled with the fast systemic clearance, suggests that systemic bleeding from intrapleural fibrinolytic use is unlikely. Intrapleural streptokinase, given in single or multiple doses, did not increase measurable systemic fibrinolysis in patients with pleural infection (41,42).
However local bleeding within the pleural cavity remains a concern. Many factors may influence the pharmacokinetics of tPA when instilled intrapleurally. tPA has a high molecular weight (>65,000) which may prohibit passive diffusion across the pleural membrane and therefore it is most likely cleared via lymphatic drainage. The pleural fluid pH and protein contents vary among patients and may alter the lipophilicity and protein binding of tPA. Therefore it is possible that the pharmacokinetics and potential activity of the drug may be different among individual patients. Using current protocols, the chest tube is opened to free drainage within 60 minutes after intrapleural instillation of tPA. Any drug that has not cleared from the pleural space should then be removed before subsequent dosing. For these reasons, all large clinical studies have found low incidences of bleeding after intrapleural fibrinolytic therapy (7,9,17).
Induction of large volumes of pleural fluid has been described as a feature following intrapleural administration of streptokinase and tPA (22,29). In 107 patients with pleural infection, the volume of pleural fluid drained increased by a striking 6.8 fold following instillations of tPA and DNase (17). The volume of fluid drained exceeded the estimated volume of pleural fluid present on imaging before treatment, suggesting that tPA/DNase stimulated pleural fluid production. Lansley et al. showed in mice that increased pleural fluid formation is a class-effect of fibrinolytics after their intrapleural administration. The fluid production was mediated by monocyte chemotactic protein (MCP-1), and could be significantly reduced with the use of different antagonists that inhibit MCP-1 activities (43). Whether the fluid production contributes to the clinical benefits of intrapleural fibrinolytic therapy, and if so to what extent, remains to be established.
Recombinant human DNase
DNase is a naturally occurring nuclease involved in the degradation of extracellular DNA following apoptosis and bacterial lysis in humans. Dornase alpha, or rhDNase, is produced by recombinant technology and is identical to endogenous human DNase. It is licensed for use as a nebulised mucolytic in cystic fibrosis (CF) patients. In CF it reduces the viscoelasticity and adhesiveness of sputum by cleaving extracellular DNA from degraded bacteria and leucocytes, separating it from its proteins.
In empyema, it is believed that the increased viscosity of pus arises from the high deoxyribonucleoprotein content from leucocyte degradation (9,26,29,44), and thus intrapleural DNase may serve to thin pus and improve its drainage. Light et al. demonstrated that the combination of streptokinase and streptodornase was needed to liquefy purulent empyema pus from rabbits. This effect was not reproduced with streptokinase alone (26,27). The early crude streptococcal extracts reported to be effective in empyema contained streptokinase as well as streptococcal DNase. The latter is not present in modern day purified streptokinase preparations. This difference may explain the lack of benefits of streptokinase alone in MIST-1, and led to the MIST-2 which examined the combination use of tPA and DNase.
Biofilm formation has been described in many common respiratory bacteria, and DNase has been shown to interfere with this matrix, potentially enhancing the effect of antibiotics (45). The formation of biofilms within the pleural space is yet to be demonstrated, therefore this proposed mechanism in pleural infection remain unclear and warrants further investigation. Treatment with DNase alone may not always be favorable. When DNase treatment was extended from CF to non-CF bronchiectatic patients, a randomized controlled trial found detrimental results (decline in FEV1 and increased exacerbations) (46). It is speculated that DNase potentially interrupts bacterial biofilms on airway surfaces and liberate the bacteria which can worsen infection and outcomes.
Nebulized DNase is well tolerated. Reported adverse effects include pharyngitis, chest pain, bronchospasm, rash and dyspnoea. Systemic complications are rare as the absorption following nebulisation is minimal (47). Small studies of intravenous DNase in humans suggest that DNase is rapidly metabolised by proteases with an elimination half life of 3-4 hours (48,49). The pharmacokinetics of DNase in the pleural space is unknown.
Combined tPA with DNase for pleural infection
The combined use of tPA and DNase has been shown to have synergistic actions in an animal model of empyema (29), and subsequent clinical trials (9). The original hypothesis was that the two agents work synergistically: tPA breaks down fibrinous septations within the pleural space to release pockets of infected pleural fluid whilst DNase reduces fluid viscosity thus allowing more complete drainage. This concept is almost certainly over-simplistic.
The interactions of tPA and DNase on the key cellular components in an infected pleural cavity, namely the residential mesothelial cells and the recruited inflammatory cells, are essentially unknown. Whether the drugs have direct effects on bacterial clearance and interactions with antimicrobials remains to be seen.
Because of this paucity of data, the common protocol used for intrapleural administration of tPA and DNase, is largely based on empirical ‘best guesses’ (9). The optimal dosage, frequency, and even order of administration, and whether the drugs can be mixed as a single instillation etc., are questions that need to be addressed.
Clinical evaluation
It’s important to recognise that pleural infection can usually be adequately treated with antibiotics and chest tube drainage alone without needing surgery. In the placebo arm of MIST-1 (7), only 14% (32/211) of the patients failed the above approach and required surgical treatment. Similarly, in MIST-2 (9) only 16% (8/51) failed conservative management. Routinely subjecting all patients to surgery therefore appears to be unnecessary. The precise role of surgery remains uncertain without strong evidence to guide when and in whom it should be employed. Nonetheless, when drainage is inadequate and there is ongoing sepsis, surgery remains a key intervention.
The main drawback of surgery is that it is restricted to those who have relatively low peri-operative risk, thus excluding many elderly patients and those with multiple comorbidities. Reported benefits of outcome from surgery are confounded by selection bias. The magnitude of the bias was highlighted in a retrospective analysis of 4,424 patients from Washington State, USA (3), of which 51.6% underwent surgery. Those surgically managed were significantly younger by almost 10 years (mean age 52.9 vs. 61.5 years) and had a considerably lower comorbidity index (3) than those treated conservatively.
Additionally, video assisted thoracoscopic surgery (VATS), and certainly thoracotomy, have associated morbidity. Furrer et al. (11) reported that chronic intercostal neuralgia occurs in up to 44% of patients 6 months post-thoracotomy. In an earlier series, 9% of patients suffered chronic post-thoracotomy pain severe enough to require daily analgesia, nerve blocks, acupuncture or pain specialist input (50). Although VATS has lower analgesia requirements and a shorter length of hospital stay as compared with thoracotomy, a third of patients still experience persistent pain or discomfort at the operation site after 3 to 18 months (11). Furthermore, close to a fifth of VATS procedures require conversion to open thoracotomy (51), thus increasing postoperative morbidity.
For these reasons, efforts have focused on identifying a minimally-invasive intrapleural treatment as an alternative to surgery. The ideal intrapleural therapy needs to be effective, safe and affordable.
Clinical evidence of efficacy
Combination tPA/DNase therapy has now been shown to satisfy the above criteria and is a viable alternative to surgical intervention.
The MIST-2, published in 2011, was a randomized, double-blind, placebo-controlled trial involving 196 adult patients (mean age 59 years, 72% men) with pleural infection (9). Patients were randomized to receive tPA plus placebo, DNase plus placebo, both agents, or double placebos. Combined intrapleural tPA/DNase resulted in a significantly greater reduction in radiographic pleural opacity, lower surgical referral rate and decreased length of hospital stay compared with placebo. The primary outcome was the absolute change in the pleural opacity on a frontal chest radiograph between days 1 and 7. The mean (SD) reduction in pleural opacity was greater with tPA/DNase than with placebo: expressed as 29.5% (23.3) vs. 17.2% (19.6) of the hemithorax on CXR. In contrast, tPA or DNase alone did not improve radiographic clearance.
Most (96%) of the 48 patients treated with tPA and DNase were successfully managed without surgery. Combination therapy was associated with a reduced duration of hospitalization, by 6.7 days vs. placebo. Overall mortality was low and there were no differences among the groups.
It is important to emphasize that only the combination therapy was effective; tPA or DNase alone offered no significant benefits. Indeed the DNase alone group had more surgical referrals than placebo. Even though only 48 patients in the trial received tPA/DNase, these results have attracted strong interest and many units worldwide have started employing the treatment.
In the MIST-2 protocol, intrapleural treatment was given at the time of diagnosis of pleural infection. As shown in the placebo group, many of these patients were successfully treated with antibiotics and fluid drainage alone, raising the question whether tPA/DNase should be considered specifically in those who failed conventional therapy. The safety profile of the treatment also requires validation.
Our recent multicentre open-label series evaluated the “real life” application of tPA/DNase treatment in 107 unselected patients with (mostly community-acquired) pleural infection (17). In 84% of patients, tPA/DNase was initiated as a “rescue therapy” >24 hours after chest drain insertion when the patient failed to improve. In addition, three quarters of the patients had ≥1 comorbidity and up to 15% had major life-limiting diseases (e.g., cancer or end stage renal failure)—patients who are often poor candidates for surgery.
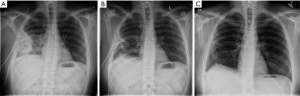
In this cohort of patients who would usually be considered for surgery, except for their comorbidity, 92.3% were successfully managed by tPA/DNase. No patients died from pleural infection. The median hospital stay from first intrapleural treatment dose was 10 (IQR, 6-17) days. Pleural fluid drainage increased from 250 mL (median) in the preceding 24 hours to a cumulative amount of 2,475 mL over the 72 hours following commencement of intrapleural therapy. A corresponding reduction in C-reactive protein and clearance of pleural opacity on chest radiographs (from 35% to 14% of the hemithorax) were observed (Figure 1).
These studies provide confidence in the efficacy of intrapleural tPA/DNase as a therapeutic option and a practical approach to their use is proposed in Table 1.
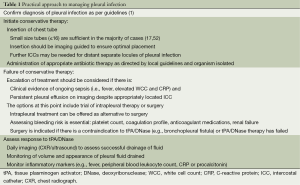
Full table
Safety
From the limited published data on clinical use of tPA/DNase of less than 160 reported cases, the treatment appears safe. Major adverse events in MIST-2 were not significantly different between the tPA/DNase and placebo groups (9). Chest pain is the most frequent side effect whereas bleeding, though uncommon, remains the biggest concern among clinicians and patients.
Chest pain, requiring initiation or escalation of opioid analgesia, occurred in ~20% of the 107 patients we reported. The pain typically occurs with the administration of the first dose of tPA treatment (17); its cause is unclear and may be multifactorial. It is possible that tPA provokes inflammatory changes in an already inflamed pleural cavity. Typically a dose of tPA stimulates the accumulation of >500 mL of pleural fluid. This sudden expansion of fluid within 60 minutes and the lysis of adhesions may generate pain. In ~4% of patients the pain was severe enough to terminate treatment (17).
Production of a large volume of hemorrhagic pleural fluid is a common observation after intrapleural administration of fibrinolytics, and often provokes anxiety. Significant pleural bleeding, defined as a reduction of haematocrit necessitating blood transfusion, is rare. In MIST-2 there were two intrapleural hemorrhages and one episode of hemoptysis in the tPA/DNase treated group (n=48), and no serious adverse events at all in the 48 patients who received tPA only (9). In our series significant pleural bleeding occurred in only 2 of 107 (1.8%) patients given tPA/DNase; both had underlying bleeding risks. There were no episodes of haemoptysis or gastrointestinal bleeding (17).
Other studies of single agent intrapleural tPA use also supported its safety. No major bleeding events were reported from 120 patients with pleural effusions of various etiologies who received 3-4 doses of intrapleural tPA (10-100 mg) daily (53). Another two studies (54,55), with 45 patients combined, also found no major bleeding complications. Isolated cases of pleural bleeding have been reported in patients treated with intrapleural tPA which were receiving concurrent anticoagulation or had end stage renal diseases (56-58).
We recommend that response to therapy should be assessed daily. Lack of clinical improvement should trigger searches for alternative source of infection (e.g., lung abscess) or residual pleural collections (which may require additional chest tube drainage). Conversely, if the pleural collection is evacuated, further doses of tPA/DNase will not be necessary. Monitoring the serum haematocrit levels can be helpful in detecting significant blood loss through induced hemorrhagic pleural fluid production. Cessation of therapy and supportive measures (e.g., transfusion) are adequate in managing most cases of fibrinolytic-induced pleural bleeding.
Future research
As published data of tPA/DNase use are still limited, many questions remain.
Whether tPA/DNase should be applied to all patients as soon as pleural infection is diagnosed or be reserved as “rescue therapy” for those who do not respond to antibiotics and fluid drainage alone is debatable. The MIST-2 data suggested that initiation of treatment significantly shortened hospitalization which argued for its use as a routine. On the other hand, treatment carries costs and potential risks (albeit uncommon) and some would argue for their use only as “rescue”.
Direct comparison of tPA/DNase versus surgery has not been performed though the high success rate and good safety profile of the former has already made it a standard treatment in many centres. No predictors of failure to treatment or development of complications from tPA/DNase exist.
Contraindications of intrapleural tPA/DNase are not well defined. High bleeding risks and/or presence of bronchopleural fistula are the commonest contraindications. To date, the regimen has only been used in adults and cannot be recommended in children, and specific patient groups (e.g., pregnant women). A randomized trial is currently underway to assess the treatment’s efficacy and safety in the paediatric population in Canada.
The dosing regimen for intrapleural tPA/DNase used in MIST2 and in our series was empirically based on early case reports. Future research should aim to optimize dosing regimes. Understanding the mechanistic actions of tPA and DNase may allow refinement of treatment and knowledge that may benefit infections of other sites.
As the incidences of serious complications from tPA/DNase are low, long-term multi-centre collaborations are needed to adequately document potential complications and their best management. Cost analysis data will be of interest.
Acknowledgements
Funding: YCG Lee is a National Health & Medical Research Council (NHMRC) Career Development Fellow and receives project grant funding from the NHMRC, New South Wales Dust Disease Board (DDB), Sir Charles Gairdner Research Advisory Committee, LIWA Westcare Alan King Grants and the Cancer Council of Western Australia.
Disclosure: YCG Lee was a coinvestigator of the MIST-2 study which was funded in part by an unrestricted grant from Roche UK.
References
- Davies HE, Davies RJ, Davies CW, et al. Management of pleural infection in adults: British Thoracic Society Pleural Disease Guideline 2010. Thorax 2010;65 Suppl 2:ii41-53. [PubMed]
- Grijalva CG, Zhu Y, Nuorti JP, et al. Emergence of parapneumonic empyema in the USA. Thorax 2011;66:663-8. [PubMed]
- Farjah F, Symons RG, Krishnadasan B, et al. Management of pleural space infections: a population-based analysis. J Thorac Cardiovasc Surg 2007;133:346-51. [PubMed]
- Finley C, Clifton J, Fitzgerald JM, et al. Empyema: an increasing concern in Canada. Can Respir J 2008;15:85-9. [PubMed]
- Shen HN, Lu CL, Li CY. Epidemiology of pleural infections in Taiwan from 1997 through 2008. Respirology 2012;17:1086-93. [PubMed]
- Søgaard M, Nielsen RB, Nørgaard M, et al. Incidence, length of stay, and prognosis of hospitalized patients with pleural empyema: a 15-year Danish nationwide cohort study. Chest 2014;145:189-92. [PubMed]
- Maskell NA, Davies CW, Nunn AJ, et al. U.K. Controlled trial of intrapleural streptokinase for pleural infection. N Engl J Med 2005;352:865-74. [PubMed]
- Bender JM, Ampofo K, Sheng X, et al. Parapneumonic empyema deaths during past century, Utah. Emerg Infect Dis 2009;15:44-8. [PubMed]
- Rahman NM, Maskell NA, West A, et al. Intrapleural use of tissue plasminogen activator and DNase in pleural infection. N Engl J Med 2011;365:518-26. [PubMed]
- Birmingham AL. Hippocrates. Aphorism 44. In: Adams LB. eds. The genuine works of Hippocrates: the classics of surgery. Delran: Gryphon Editions, 1985:768-71.
- Furrer M, Rechsteiner R, Eigenmann V, et al. Thoracotomy and thoracoscopy: postoperative pulmonary function, pain and chest wall complaints. Eur J Cardiothorac Surg 1997;12:82-7. [PubMed]
- Bouros D, Schiza S, Patsourakis G, et al. Intrapleural streptokinase versus urokinase in the treatment of complicated parapneumonic effusions: a prospective, double-blind study. Am J Respir Crit Care Med 1997;155:291-5. [PubMed]
- Davies RJ, Traill ZC, Gleeson FV. Randomised controlled trial of intrapleural streptokinase in community acquired pleural infection. Thorax 1997;52:416-21. [PubMed]
- Bouros D, Schiza S, Tzanakis N, et al. Intrapleural urokinase versus normal saline in the treatment of complicated parapneumonic effusions and empyema. A randomized, double-blind study. Am J Respir Crit Care Med 1999;159:37-42. [PubMed]
- Diacon AH, Theron J, Schuurmans MM, et al. Intrapleural streptokinase for empyema and complicated parapneumonic effusions. Am J Respir Crit Care Med 2004;170:49-53. [PubMed]
- Thommi G, Shehan JC, Robison KL, et al. A double blind randomized cross over trial comparing rate of decortication and efficacy of intrapleural instillation of alteplase vs placebo in patients with empyemas and complicated parapneumonic effusions. Respir Med 2012;106:716-23. [PubMed]
- Piccolo F, Pitman N, Bhatnagar R, et al. Intrapleural tissue plasminogen activator and deoxyribonuclease for pleural infection. An effective and safe alternative to surgery. Ann Am Thorac Soc 2014;11:1419-25. [PubMed]
- Tillett WS, Sherry S. The effect in patients of streptococcal fibrinolysin (streptokinase) and streptococcal desoxyribonuclease on fibrinous, purulent, and sanguinous pleural exudations. J Clin Invest 1949;28:173-90. [PubMed]
- Mitchell ME, Alberts WM, Chandler KW. Intrapleural streptokinase in management of parapneumonic effusions. Report of series and review of literature. J Fla Med Assoc 1989;76:1019-22. [PubMed]
- Rosen H, Nadkarni V, Theroux M, et al. Intrapleural streptokinase as adjunctive treatment for persistent empyema in pediatric patients. Chest 1993;103:1190-3. [PubMed]
- Taylor RF, Rubens MB, Pearson MC, et al. Intrapleural streptokinase in the management of empyema. Thorax 1994;49:856-9. [PubMed]
- Strange C, Allen ML, Harley R, et al. Intrapleural streptokinase in experimental empyema. Am Rev Respir Dis 1993;147:962-6. [PubMed]
- Moulton JS, Moore PT, Mencini RA. Treatment of loculated pleural effusions with transcatheter intracavitary urokinase. AJR Am J Roentgenol 1989;153:941-5. [PubMed]
- Lee KS, Im JG, Kim YH, et al. Treatment of thoracic multiloculated empyemas with intracavitary urokinase: a prospective study. Radiology 1991;179:771-5. [PubMed]
- Lee YC. Ongoing search for effective intrapleural therapy for empyema: is streptokinase the answer? Am J Respir Crit Care Med 2004;170:1-2. [PubMed]
- Simpson G, Roomes D, Heron M. Effects of streptokinase and deoxyribonuclease on viscosity of human surgical and empyema pus. Chest 2000;117:1728-33. [PubMed]
- Light RW, Nguyen T, Mulligan ME, et al. The in vitro efficacy of varidase versus streptokinase or urokinase for liquefying thick purulent exudative material from loculated empyema. Lung 2000;178:13-8. [PubMed]
- Simpson G, Roomes D, Reeves B. Successful treatment of empyema thoracis with human recombinant deoxyribonuclease. Thorax 2003;58:365-6. [PubMed]
- Zhu Z, Hawthorne ML, Guo Y, et al. Tissue plasminogen activator combined with human recombinant deoxyribonuclease is effective therapy for empyema in a rabbit model. Chest 2006;129:1577-83. [PubMed]
- Idell S, Girard W, Koenig KB, et al. Abnormalities of pathways of fibrin turnover in the human pleural space. Am Rev Respir Dis 1991;144:187-94. [PubMed]
- Alemán C, Alegre J, Monasterio J, et al. Association between inflammatory mediators and the fibrinolysis system in infectious pleural effusions. Clin Sci (Lond) 2003;105:601-7. [PubMed]
- Philip-Joët F, Alessi MC, Philip-Joët C, et al. Fibrinolytic and inflammatory processes in pleural effusions. Eur Respir J 1995;8:1352-6. [PubMed]
- Chung CL, Chen CH, Sheu JR, et al. Proinflammatory cytokines, transforming growth factor-beta1, and fibrinolytic enzymes in loculated and free-flowing pleural exudates. Chest 2005;128:690-7. [PubMed]
- Jantz MA, Antony VB. Pleural fibrosis. Clin Chest Med 2006;27:181-91. [PubMed]
- Maskell NA, Gleeson FV. Images in clinical medicine. Effect of intrapleural streptokinase on a loculated malignant pleural effusion. N Engl J Med 2003;348:e4. [PubMed]
- Squire IB, Lawley W, Fletcher S, et al. Humoral and cellular immune responses up to 7.5 years after administration of streptokinase for acute myocardial infarction. Eur Heart J 1999;20:1245-52. [PubMed]
- Lawley WJ, Fletcher S, Squire IB, et al. T-ceIl recognition of discrete regions of the thrombolytic drug streptokinase. Clin Sci (Lond) 2000;99:239-46. [PubMed]
- Popowicz N, Piccolo F, Shrestha R, et al. Two sequential tPA/DNase courses for noncommunicating loculated collections in pleural infection. Respirol Case Rep 2014;2:87-9. [PubMed]
- Askari AT, Messerli AW, Lincoff AM. eds. Management Strategies in Antithrombotic Therapy. Hoboken: John Wiley and Sons, 2007.
- Berkowitz SD, Granger CB, Pieper KS, et al. Incidence and predictors of bleeding after contemporary thrombolytic therapy for myocardial infarction. The Global Utilization of Streptokinase and Tissue Plasminogen activator for Occluded coronary arteries (GUSTO) I Investigators. Circulation 1997;95:2508-16. [PubMed]
- Berglin E, Ekroth R, Teger-Nilsson AC, et al. Intrapleural instillation of streptokinase. Effects on systemic fibrinolysis. Thorac Cardiovasc Surg 1981;29:124-6. [PubMed]
- Davies CW, Lok S, Davies RJ. The systemic fibrinolytic activity of intrapleural streptokinase. Am J Respir Crit Care Med 1998;157:328-30. [PubMed]
- Lansley SM, Cheah HM, Varano Della Vergiliana JF, et al. Tissue Plasminogen Activator Potently Stimulates Pleural Effusion via an MCP-1 Dependent Mechanism. Am J Respir Cell Mol Biol 2014. [Epub ahead of print]. [PubMed]
- Dikensoy O, Zhu Z, Na MJ, et al. Intrapleural heparin or heparin combined with human recombinant DNase is not effective in the treatment of empyema in a rabbit model. Respirology 2006;11:755-60. [PubMed]
- Hall-Stoodley L, Nistico L, Sambanthamoorthy K, et al. Characterization of biofilm matrix, degradation by DNase treatment and evidence of capsule downregulation in Streptococcus pneumoniae clinical isolates. BMC Microbiol 2008;8:173. [PubMed]
- O'Donnell AE, Barker AF, Ilowite JS, et al. Treatment of idiopathic bronchiectasis with aerosolized recombinant human DNase I. rhDNase Study Group. Chest 1998;113:1329-34. [PubMed]
- Aitken ML, Burke W, McDonald G, et al. Recombinant human DNase inhalation in normal subjects and patients with cystic fibrosis. A phase 1 study. JAMA 1992;267:1947-51. [PubMed]
- Genetech. Pulmozyme Prescribing Information. 2014. Cited 16/12/2014. Available online: http://www.gene.com/download/pdf/pulmozyme_prescribing.pdf
- Davis JC Jr, Manzi S, Yarboro C, et al. Recombinant human Dnase I (rhDNase) in patients with lupus nephritis. Lupus 1999;8:68-76. [PubMed]
- Dajczman E, Gordon A, Kreisman H, et al. Long-term postthoracotomy pain. Chest 1991;99:270-4. [PubMed]
- Landreneau RJ, Keenan RJ, Hazelrigg SR, et al. Thoracoscopy for empyema and hemothorax. Chest 1996;109:18-24. [PubMed]
- Rahman NM, Maskell NA, Davies CW, et al. The relationship between chest tube size and clinical outcome in pleural infection. Chest 2010;137:536-43. [PubMed]
- Thommi G, Nair CK, Aronow WS, et al. Efficacy and safety of intrapleural instillation of alteplase in the management of complicated pleural effusion or empyema. Am J Ther 2007;14:341-5. [PubMed]
- Zuckerman DA, Reed MF, Howington JA, et al. Efficacy of intrapleural tissue-type plasminogen activator in the treatment of loculated parapneumonic effusions. J Vasc Interv Radiol 2009;20:1066-9. [PubMed]
- Froudarakis ME, Kouliatsis G, Steiropoulos P, et al. Recombinant tissue plasminogen activator in the treatment of pleural infections in adults. Respir Med 2008;102:1694-700. [PubMed]
- Anevlavis S, Archontogeorgis K, Tzouvelekis A, et al. Intrapleural r-tPA in association with low-molecular heparin may cause massive hemothorax resulting in hypovolemia. Respiration 2011;81:513-6. [PubMed]
- Gervais DA, Levis DA, Hahn PF, et al. Adjunctive intrapleural tissue plasminogen activator administered via chest tubes placed with imaging guidance: effectiveness and risk for hemorrhage. Radiology 2008;246:956-63. [PubMed]
- Goralski JL, Bromberg PA, Haithcock B. Intrapleural hemorrhage after administration of tPA: a case report and review of the literature. Ther Adv Respir Dis 2009;3:295-300. [PubMed]


