According to previous reports, the early enhancement is the
maximal enhancement obtained within the first 3 minutes after
contrast injection (
19). When taking curves, in order to avoid
partial volume effect or inclusion of necrotic tumoral areas, the
use of small ROIs positioned on subjectively recognized areas
of maximal constrast enhancement is proposed. That study (
19)
demonstrates that the type, the dimensions and the positioning
of the ROI considerably influences the calculation of the early
contrast enhancement which is an important parameter of the
dynamic behavior of a breast lesion. The general effect is that
as the size of ROI decreases on the maximal enhancement, it
increases sensitivity and it reduces specificity and the number
of uncertain curves. Vice versa, as the size of ROI increases, it
reduces sensitivity and increases specificity and the number of
uncertain curves (
19). This is due to different possibilities that
can take place by reducing or enlarging the ROI: (I) reducing
the ROI to a small target on the most vascularized area in a
malignant tumor (increasing sensitivity), (II) reducing the ROI
to a small target on the most vascularized area in a benign lesion
(decreasing specificity), (III) including internal (necrotic or lowvascularized)
and/or external (non-neoplastic) areas in presence
of a malignant tumor (decreasing sensitivity) and (IV) including
internal (low-vascularized) and/or external (normal gland or fat)
areas in presence of a benign tumor (increasing specificity) (
19).
However, the factors mentioned above may significantly
influence the kinetic curve analysis, which is based on the
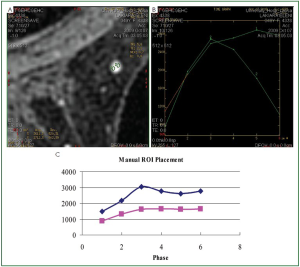
three patterns of signal-to-time curve. Curve type I is defined
as a pattern of continuous increase in signal intensity. This
enhancement pattern is usually associated with benign
findings (83% benign, 9% malignant) (
20). Its sensitivity and
specificity for indication of a benign lesion are 52.2% and
71.0%, respectively (
21). Curve type II is defined as the plateau
pattern of enhancement, in which an initial increase in signal
intensity is followed by a flattening of the enhancement curve.
This pattern has a sensitivity of 42.6% and specificity of 75% for the detection of malignancy. Schnall and colleagues reported
that radiologists in a multi-institutional trial described the
enhancement kinetics as persistent (plateau) in 45% of lesions
that proved to be cancers (
21). Type III curve represents the
washout pattern of enhancement and it involves an initial
increase and subsequent decrease in signal intensity (specificity
90.4%, sensitivity 20.5%) (
21). Schnall and colleagues reported
that 76.0% of lesions with washout curves were proved to be
malignant (
22). Both type II and type III curves should be
considered suggestive of malignancy (
1).
The inclusion of external non-neoplastic breast gland or fatty
tissue in the calculation of the kinetic curves using large ROIs
may be the result of inaccurate measurement not only in the
same slice (when the ROI includes pixels that are external from
the lesion) but also in between different slices (when a small
lesion is depicted with a partial volume effect, probably when
the size of the lesion is comparable with the slice thickness).
The former type of error is easier to be recognized and may be minimized when the ROI is placed very carefully.
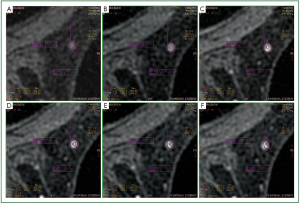
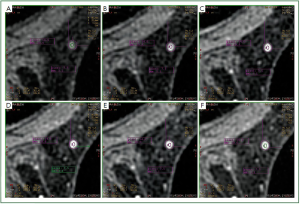
In our study, we observed that in a number of cases of MRM
lesions, there was a movement of the ROI in different phases of
the dynamic study of a lesion. More specifically, although the
ROI was located in a specific region of the lesion at the third
phase of the dynamic study, in other phases the ROI was found
to be located in another region of the lesion or even outside the
lesion. It has to be mentioned that this was the first time that the
magnitude of this problem was quantified and its clinical impact
was evaluated. Additionally, in this study, the effects of motion
were associated with factors such as the size of breast and the
density of breast.
Magnetic Resonance Mammography requires a high spatial
resolution to resolve morphologic and architectural details of
even small tumors. At the same time, fast imaging is required to
account for the transient enhancement of breast lesions. This is the
“temporal versus spatial dilemma” that current breast MR imaging
protocols face. Most of the researchers agree that the temporal
resolution should be between 1-2 min with high spatial resolution
compared to a more fast protocol of 40 sec temporal resolution
and a little lower spatial resolution. In 3T MRI, the signal to noise
ratio (SNR) is double compared to 1.5T achieving increased
spatial resolution resulting in pixels of small size. We suspect that
we could observe this kinetic error in 3T MRI because it becomes
more pronounced when small lesions are examined due to the
small pixel size and the increased signal-to-noise ratio.
According to Kuhl (
23), 3D sequences have increased
blurring compared to 2D sequences, especially in the subtraction
images. In our protocol, a 3D sequence is used with high
spatial resolution and an increased blurring is observed in the
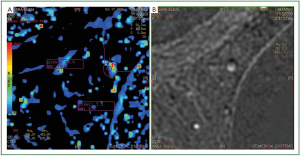
subtraction images. Furthermore, subtraction images of lesions
with kinetic errors have artifacts in the region where the motion
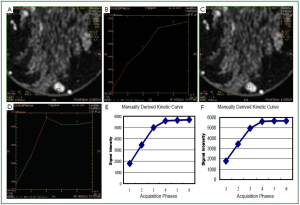
takes place (
Figure 4B). It is expected that the subtraction image
will have more blurring and a higher probability of showing an
artifact because, due to motion, it is not the same tissue that is
subtracted in the images of the different phases.
Furthermore, in the same region that we observed the artifact
at the subtraction images, we also observed both increased
enhancement and increased washout in the mapping images
(
Figure 4A). It is straightforward that, in order to have correct
results in the subtracted and mapping images, we should not
have motion of the patient involved.
Overall, there are three ways for identifying a potential kinetic
error: (I) ROI placing, (II) image subtraction and (III) image
mapping. Its observation in all three of them could ensure that
there is an error, which will also introduce kinetic curve errors.
In recent years, there is a number of image registration
methods that have been suggested for reducing kinetic errors
(
13,
24,
25). However, the non-rigid, inhomogeneous, anisotropic
and temporally changing nature of breast tissue makes breast
image registration a challenging task. Breast image registration
methods are a compromise among accuracy, precision, reliability,
robustness, and issues-like automation, interactivity, speed, and
patient-friendliness (
13). Despite these constraints, we propose
the integration of such image registration tools in the existing
breast image processing systems, especially in 3T where the
impact of these kinetic errors is more pronounced.
Consequently, it is recommended that for the correct
production of the kinetic curves, it should be examined if the
ROI’s placement remains at the same region of the lesion in all
the series of the dynamic phase. In order to achieve this goal, the
position of the ROI should be estimated from stable reference
point (e.g., skin, sternum, muscle). Regarding the accuracy of
the results produced by the manual method, the procedure was
repeated by different users for a number of lesions and the results
were found to be identical.
When the ROI is placed in the mapping images, it should
also be examined if the position of the ROI coincides with the
true area to be measured. Due to motion, the mapping images
are more sensitive to false positive results because they will
show increased signal intensity in the phase of enhancement or
increased decay in the later phases (
Figure 4). Working on the
mapping images it is easier to place a ROI in the region with the
highest enhancement due to the fact that this region is colored
(red color). However, as we have already stated, the errors that
we measured were the same irrespectively whether we placed
the ROI using the mapping image or the DCE image. On the
contrary, we tested all the different techniques (placing the ROI
in the mapping image as well as in the DCE image) in order to
avoid bias. Especially, in the mapping image, the red colored
region was the one that was more prone to motion.
In our study we found that a change of the enhancement
kinetic curve type was found in a considerable proportion of
the cases (21.5%). This observation was most pronounced in
the small and non-dense breasts and in this category most of the
errors were observed in the left breasts. The fact that most of the
curve errors were observed in small and non-dense breasts led us
to suspect that the source of those errors may stem from non firm
immobilization of the breasts due to the large size of the coil that
was used. Large breasts usually have a more firm immobilization
for the type of the coils used. In these cases, the lesions did not
show kinetic errors. We propose the performance of prospective
studies using coils of different sizes to prove if there can be any
correlation between the reduction of curve errors and the size
of the coil. Based on our experience, such a coil could have
more than two depressors in order to achieve a more firm and
homogeneous immobilization.
Based on our observation that left breasts were more prone
to errors in the kinetic curves, we assume that these errors stem
from the cardiac motions. In the future, it should be possible
to perform dynamic studies using breathing or cardiac gating
in case that the technical errors observed are characterized by periodic patterns. However, in 3.0T MRI the increased signal/
noise ratio combined with a reduced spatial resolution could
keep the dynamic sequence time in clinically acceptable limits.
The manufacturers have developed tools for image registration
but those tools are generic for all types of imaging examinations
and they are usually used to eliminate the presence of motion or
perform a correspondence between different sequences in order
to apply a subtraction technique and there is movement of the
patient. Furthermore, many imaging systems do not offer image
registration tools (in their standard version or at all), which
makes the use of a manual technique inevitable.
However, although a number of image registration techniques
have been developed to account for the effects of motion, their
efficiency and accuracy are limited by the complexity of the task
to correct the presence of motion. So, although the use of image
registration techniques offers speed and convenience at the same
time they are subject to limitations. For example, the choice of
algorithm magnitude of motion and timing of the motion are
each shown to influence estimated pharmacokinetic parameters
even when motion magnitude is small.
To overcome the problem imposed by motion in DCEMRM,
it is necessary to correct patient motion by deformable
registration, before the acquisition of the DCE-MRI. However,
the dramatic contrast change over time (especially between the
precontrast and postcontrast images) makes the conduction of
deformable registration of DCE-MR images difficult (
26). Most
existing methods typically register each postcontrast image onto
the precontrast image independently, without considering the
dynamic contrast change after agent uptake. This could lead to
the inconsistency among the aligned postcontrast images in the
precontrast image space, which will eventually result in worse
performance in cancer detection (
26). Similarly, Melbourne
et al. (
27) performed an analysis of the effect of registration
completeness and timing of subject motion, which revealed that
a higher degree of motion increases model-fit residuals. Motion
at a time when contrast arrives is particularly undesirable and
the choice of registration algorithm matters, even when motion
artifacts are small (
27).
Factors such as the Ktrans should be incorporated in the
evaluation of the tumor histologic grade. Patankar
et al. (
6)
reported that the Ktrans factor showed good discriminative
power in distinguishing between low- and high-grade tumors
with diagnostic sensitivity and specificity >90%. Similarly, Ah-
See
et al. (
28) reported that Changes in breast tumor microvessel
functionality as depicted by DCE-MRI early on after starting
anthracycline-based neoadjuvant chemotherapy can predict final
clinical and pathologic response with the Ktrans being the best
predictor of pathologic nonresponse. Liu
et al. (
29) reported
that indicators of a vascular response, such as the volume
transfer constant (Ktrans) were calculated to assess the effect
of treatment on tumor vascular function and they concluded
that vascular response measured using DCE-MRI seems to be a
useful indicator of drug pharmacology, and additional research
is needed to determine if it is a suitable marker for predicting
clinical activity. Finally, Springer
et al. (
30) reported argue that
the ΔKtrans subtraction minimizes/eliminates many other
systematic DCE-MRI quantification errors. However, none of
all these studies investigated the impact of motion and proposed
way for its elimination.
The present study, involves a manual approach, which is more
accurate in order to demonstrate the extent of the uncertainty
due to motion and be used as a benchmark for evaluating
different automatic image registration algorithms and software.
The presented technique can be easily applied without having
as a prerequisite any special technology beyond the standard
one and it may be time consuming only when there are many
pathologies involved in a breast case. However, in cases involving
many pathologies, the use of image registration tools may also
be complex and time consuming because the magnitude of
motion changes with the position of the breast and time. So,
the different image registration algorithms have to be evaluated
in such situations regarding their ability to reduce or even
eliminate motion effects in the derivation of kinetic curves.
The development of computational algorithms for automatic
ROI placement based on the distances of the ROI from stable
reference points (e.g., skin, sternum, muscle) is proposed. These
algorithms should reduce or even eliminate the effects of the
breast image registration constraints.