
Alternative methods of lymph node staging in lung cancer: a narrative review
Introduction
Lung cancer is one of the most common cancers in the world. According to statistics, in the United States, nearly 1.8 million people are diagnosed with lung cancer each year only, and more than 600,000 people died because of this malignancy in 2019 (1). According to the literature, the 5-year overall survival (OS) rate of patients with non-small cell lung cancer (NSCLC), accounting for approximately 80% of all cases of lung cancer, is 18% (2). The current clinical nodal (cN) and pathological nodal (pN) NSCLC classifications are based only on the anatomical location of nodal metastases. The nodal stages are used to predict the 5-year OS rates both in clinical staging (60%, 37%, 23%, and 9% for cN0-3, respectively) and in pathological staging (75%, 49%, 36%, and 20% for pN0-3, respectively) (3). The International Association for the Study of Lung Cancer (IASLC) did not introduce any changes in nodal categorization since the 6th edition (4). Nonetheless, the current classification has some limitations. Ongoing N descriptors of the 8th Tumor Node Metastasis classification (TNM) create heterogeneous divisions of NSCLC patients due to situations such as skip N2 metastases, microscopic nodal metastases, and clinically occult pN2 disease (3,5,6). However, clinically, such anatomical staging is a valuable and easy system for making treatment decisions and is relatively visible and distinguishable in preoperative imaging and invasive nodal staging. Many alternative classifications have been suggested. In propositions for both the 7th and 8th editions of the NSCLC staging system, the IASLC suggested quantitative factors that were not ultimately adopted (3,4). In this study, we present a review of various suggested factors analysed as potential additions or successors to the current nodal staging of NSCLC. We present the following article in accordance with the Narrative Review reporting checklist (available at http://dx.doi.org/10.21037/jtd-20-1997).
Material and methods
We searched the PubMed database regarding ongoing and newly suggested lymph node descriptors in lung cancer. We used the following search terms and their combinations: “lung cancer”, “NSCLC”, “lymph node staging”, “number of positive lymph nodes”, “number of negative lymph nodes”, “number of dissected lymph nodes”, “lymph node ratio”, “log odds of positive lymph nodes”, “lymph node stations”, “skip N2 metastasis”, “lymph node zones”, and “nodal chains”. After the first search, we included additional articles retrieved from a manual search of cited references and articles that cited articles about particular classifications via a PubMed search. The inclusion criteria were as follows: original articles and articles published from 1990 to present. The exclusion criteria were as follows: no full text available and abstract/full text not in English.
Number of lymph nodes
Number of positive lymph nodes
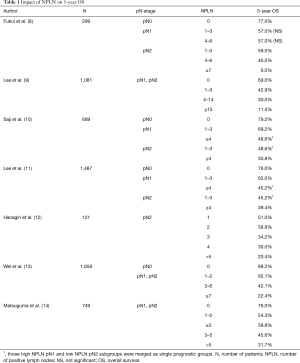
The quantity of metastatic lymph nodes is a meaningful prognostic factor. Unlike lung cancer, the number of positive lymph nodes (NPLN) has already demonstrated a crucial role in the clinical and/or pathological TNM staging of various malignancies (e.g., breast cancer). Notably, esophageal cancer staging is also based on the NPLN despite the anatomical surroundings and lymph node drainage being comparable to those of lung cancer (7). There are many studies regarding the importance and usefulness of the NPLN in NSCLC. As shown in Table 1, we summarized the impact of the NPLN on OS. Various approaches were made towards use of the NPLN in staging. Some researchers used the number of metastatic lymph nodes as an addition to ongoing pN descriptors to subdivide patients (8-12,15). Promising results were achieved. pN2 patients with a few positive lymph nodes (PLN) (range from 1 to 3) had a better survival rate than pN2 patients with more PLN (range from 4 to 6). The subdivision of pN1 patients was not significant; however, the pN2 subgroup with 1–3 PLN had a similar OS rate as the pN1 subgroup. Skip N2 metastases and single-station disease were associated with fewer PLN, indicating the complex relationship of various factors for the revision of N staging (8). The significance of pN2 subdivision and the insignificance of pN1 subdivision were similarly observed in another study (NPLN groups: 1–3, 4–14, and ≥15) (9). However, in another study, pN1 subdivision was significant. Differences between pN2 with 1–3 PLN were not significantly different from pN1 with more PLN (range from 4 to 6). The authors suggested merging these two subgroups into a single prognostic group. However, no significance between the two subgroups could be a result of the small population included in these two subdivisions (10). This combined group was also used in another study (11). Rearrangement of the IIIA and IIIB stages of NSCLC based on the NPLN, values was suggested (15). Patients with less than 3 PLN had relatively better survival than those with more than 2 PLN (12).
Full table
Other researchers suggested changing N staging completely and using the NPLN alone (13). The number of metastatic lymph nodes (groups: 0; 1–2; 3–6; and ≥7) was a better prognostic factor than the pN1 and pN2 stages, which, in this study, were statistically insignificant and worse than the NPLN in the multivariate analysis. Subdivision of the pN1 and pN2 subgroups based on the NPLN was significant; nonetheless, subdivision of the NPLN categories into pN1 and pN2 was not. This result might imply that the NPLN creates more homogeneous subgroups and that the location of metastatic lymph nodes may not be related to survival (13). Nonetheless, the higher the pN status is, the larger the NPLN, which indicates the relationship of the anatomical extent of the N stage and the NPLN (9,13).
According to the literature, the NPLN cut-off values vary between studies. Additionally, as it highly depends on proper lymphadenectomy, the minimal number of dissected lymph nodes (NDLN) must be addressed to stage patients properly. One study, for example, suggested an NPLN cut-off value of 4 and proper lymphadenectomy for a minimum of 10 dissected nodes (16).
Number of negative lymph nodes
The number of negative lymph nodes (NNLN), as the antithesis of the NPLN, may also be used to predict patient survival. The NNLN has been studied in various cancers (e.g., esophageal cancer), but only a few analyses have been performed in terms of NSCLC (17). The most recent work (n=1,019) revealed that the new classification, based on current pN categories combined with the NNLN (cut-off equal to 8), has the strongest predictive value [compared to pN alone, NPLN and even lymph node ratio (LNR)] (18). The NPLN and LNR were excluded in the multivariate analysis, unlike pN-NNLN (18). Nonetheless, in another study from 2017 (n=482), the NNLN (cut-offs equal to 10 and 30) failed to be an independent prognostic factor in the multivariate analysis, unlike the LNR (cut-offs =20% and 55%). However, researchers suggested combining the classification based on the NNLN groups with the LNR subgroups (the classification based on the opposite LNR groups with NNLN subgroups was not significant), which stratified patients better than pN (19). The NNLN alone has an impact on survival. In one analysis, OS in patients with fewer NNLN (≤15) was better than that in patients with more NNLN (>15) (P=0.002). The survival curves were similar to those generated with pN0; however, differences were observed in the pN1 and pN2 subgroups (14).
Number of dissected lymph nodes
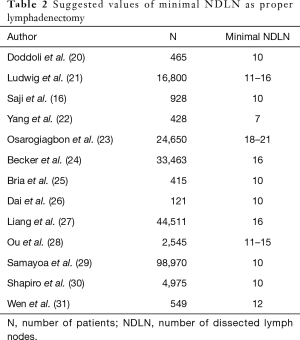
The NDLN is essentially the sum of the two parameters mentioned above (NDLN = NPLN + NNLN). Thus, the NDLN might also be used to predict patient survival. In the TNM classifications of many cancers, the minimum number of removed lymph nodes is determined (e.g., breast 6, colon 12, and stomach 16) (7). Many studies on the minimal NDLN in NSCLC have been performed. As shown in Table 2, we summarized the suggested minimal NDLN that were prognostically significant. It is possible that the number of lymph nodes is an individual characteristic of the patient and distributed as a Gaussian curve in the population. Thus, in this study, the NDLN had no impact on OS, unlike the extent of nodal metastasis (single-station vs. multi-station) (32).
Full table
Lymph node ratio
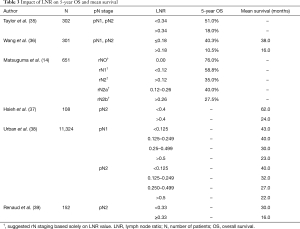
The LNR is defined as the ratio of the NPLN to the total number of all resected nodes
Full table
Many studies have aimed to determine optimal LNR values for the selection of patients who could benefit from adjuvant therapy. For example, among the pN2 groups, patients with an LNR higher than 0.50 should undergo postoperative therapy (38). Interestingly, the survival outcomes of patients with an LNR less than 0.18 were not different regardless of whether they underwent chemotherapy (36). The usefulness of the LNR was also confirmed when predicting the survival of patients who underwent neoadjuvant therapy (39) and in predicting brain metastases of NSCLC (42). The LNR could be an alternative to positron emission tomography scans according to one study. In terms of the prediction of recurrence, an LNR >0.12 was the second-best predictor after the maximum standardized uptake value (>6.5). Thus, the LNR could be a great compromise for countries where these scans are not accessible due to various reasons (43).
Despite having robust usefulness and being generally better than the NPLN, the LNR also has some limitations. Heterogeneity in this system is also possible. For instance, when 0 of 2 resected lymph nodes is positive and 0 of 17 resected lymph nodes is positive (both 0), the ratio is equal to 0. Mathematically, more situations such as these are possible (e.g., 2/2 and 14/14 are both equal to 1). The log odds of positive lymph nodes (LODDS) would discriminate such situations, as its formula would yield different results. This was proven in the work by Deng et al., where the LODDS was particularly better than the LNR in the case of an LNR equal to 0 and 1 (44). When the minimal NDLN is agreed on, situations such as those observed with the LNR could be avoided, at least partially. This was also proven in that study. The LODDS was superior to the LNR only when the NDLN was less than 10. In the case of proper lymphadenectomy, the LODDS was surpassed (44).
Log odds of positive lymph nodes
The LODDS is defined as the logarithm of the ratio of the NPLN to the NNLN.
In various studies, the LODDS was found to be a better descriptor than the LNR and current pN staging (44,46-48). Lv et al. even suggested the so-called TLM (Tumor, LODDS, Metastases) staging, which was identified as an independent risk factor, unlike the current TNM staging. This descriptor was good at diminishing heterogeneity in pN and in cases of an LNR less than 0.036 (46). The LODDS was especially better in higher-stage patients. In the case of lower stage survival, the curves for pN, the LNR and the LODDS were similar. Only the LODDS was identified as an independent risk factor in the multivariable analysis. However, in that study, only the adenocarcinoma group was included (47). Introducing the LODDS into the staging system would allow us to classify some pN1 patients into the pN2 group and pN2 patients into the pN1 group based on survival (48). As already mentioned in other classifications, the LODDS is becoming increasingly important, especially in the case of 0 PLN and an LNR equal to 1 (44). Zero PLN (thus pN0) seems to be particularly interesting because some programmes treat selected pN0 patients postoperatively because of their poor prognosis. Additionally, in the case of the LODDS, cut-off values vary between studies. In two studies, there was a single cut-off value: −1.142 (46) and 0.26 (44). In one study, there were 4 different groups based on values ranging from −2.10 to 1.74 (47). Even more groups (seven) existed in the most recent work, where values ranged from −6 to 2 (48).
In comparison to the NPLN or LNR, the LODDS seem to be the least faulty system (especially when there is no consensus on the minimal NDLN). Its usefulness is narrow due to limited data; nonetheless, as the mathematical modification of the ratio, it may be very robust. Previous descriptors are usually reported in pathological results or can be easily calculated.
Single or multiple station involvement
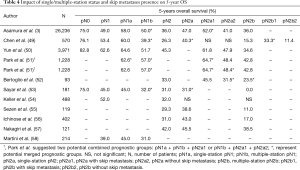
In 2015, the IASLC suggested a new subclassification of N staging in lung cancer for the 8th edition of the TNM staging system based on single/multiple station disease and skip metastases (3). Although it was not accepted, as data were not sufficient, in the future, such a system might be adapted into everyday practice. The subclassification of N descriptors is as follows: pN1 is subdivided into single (pN1a) and multiple (pN1b) stations, and pN2 is also subdivided into single (pN2a) and multiple (pN2b) stations. Further, pN2a is again subdivided into a lack of N1 involvement (pN2a1, so-called skip-N2) and with N1 involvement (pN2a2). pN3 remains intact. All of these subdivisions yielded significantly distinct groups (3). Since then, many studies have been performed to validate this classification (Table 4). Additional N2b subdivision into N2b1 and N2b2 based on skip N2 metastases might be used. N2b1 and N2b2 are significantly distinct groups. Unlike other works, the N2a1 and N2a2 subgroups showed no significant difference (49). Potential combined prognostic groups seem to vary, and based on statistical significance, researchers have suggested many possibilities (Table 4) Nonetheless, some of combinations may be the result of small samples in subgroups (50,51). Compared with other propositions, this system is worse than the LNR and NPLN (52).
Full table
Years before Asamura’s idea, many studies were made on the role of multiple and single station disease in the prognosis of NSCLC patients were performed. In some studies, the difference between pN1 subgroups was not significant, as was sometimes the case for pN2 subgroups (54). In other works, the distinction between multiple stations and a single station was significant in both N1 (53,58) and N2 (55-57) patients. Interestingly, tumor location might have an impact on single and multiple station statuses (56,59). Skip N2 metastases, as a distinct prognostic group, were also either significant or insignificant in various analyses (55,57).
Even the authors of the largest IASLC study noted limitations because most of the records were based on the Japanese population and nodal staging was based on the Naruke-Japanese nodal map (3). Currently, only the Mountain-Dresler modification of the American Thoracic Society is recommended. The recognition of each nodal station might be challenging, and errors are possible. Some stations could be indistinguishable because of their adjacency (e.g., to the paratracheal), and this could lead to an incorrect diagnosis. That is why some researchers suggested grouping nodal stations into zones (levels).
Other classifications
During propositions for the 7th edition of the TNM staging system in 2007, the IASLC suggested a system for grouping lymph node stations into zones (levels) that was not implemented and eventually discarded (4). Compared to the LNR and NPLN, zone-based staging is inferior and not as prognostic (60). Propositions for the 7th and 8th editions of the TNM staging system were combined into a single staging system by Yun et al. (based on single/multiple zones and skip metastasis). In comparison to the station-based system, the zone-based system is as equal, and some patients (7.1%) are downstaged (50).
All suggestions disregarded extranodal metastases, which are important according to the nodal chains (NC) system. Each NC consists of at least one lymph node vessel and various lymph nodes at different stations. This staging classification was studied as the ratio between positive and resected NC (61). Excluding extranodal metastases, NC are an intermediate system between zones and stations and could have a single status or multiple statuses (62).
Interestingly, when multiple-station cases are limited to a single-zone or single-chain, survival is as good as single-station cases (50,62). Unfortunately, these and other systems (e.g., systems based on a relationship between tumor location and station/zone of the involved lymph node) extend beyond the framework of our article (63).
Discussion
The minimal number of resected lymph nodes in NSCLC has not been established, and according to various studies and our opinion, it has to change. In the 8th edition of the TNM staging system, it is suggested that at least 6 lymph nodes should be resected for adequate staging; however, it is not enforced as mandatory, and it is not an appropriate number according to various studies (7). Among these studies, 10 was the most frequent minimal value of harvested lymph nodes that was prognostically significant (Table 2). More extensive lymph node resection might be even more beneficial; for instance, an NDLN equal to 16 was the best value in regard to OS, and the other best value ranged from 18 to 21, which demonstrated the best benefit for patients (21,23). Nonetheless, such numbers might not be achieved in some cases because of anatomical interindividual differences (32). Regardless, we should aim to achieve such values and, if not possible, approach such patients individually (e.g., by the LODDS) or classify and treat such patients at least as pNx. In practice, there are many possibilities for proper nodal dissection. Lymphadenectomy provides good staging, and sampling decreases the surgery time and complication rate (20,64). The lobe-specific method allows the dissection of lymph nodes according to lymphatic drainage and anatomy (65). Targeted sampling decreases the rate of the dissection of dangerous lymph nodes (e.g., station 7 and postoperative ischaemic bronchitis) (66).
The main limitation of the studies included in our review is their retrospective nature. Selection bias could be possible. Additionally, most of the studies used different inclusion or exclusion criteria (e.g., minimal NDLN).
In this review, we summarized the most important and prominent suggestions to consider as potential pN descriptors in NSCLC. The vastness of newly suggested NSCLC classifications and IASLC suggestions indicate the need for upcoming changes. As we already mentioned in our criticism of the ongoing 8th edition of the TNM staging system, heterogeneity in N descriptors is the main reason behind the need for such changes. It is necessary to determine the optimal pN classification to refer eligible patients for adequate adjuvant therapies. When considering other organs, multiple classifications are applied. The staging of esophageal cancer, an anatomically related malignancy to NSCLC, is based on the NPLN (7). Therefore, based on the reviewed papers, a quantity-based pN descriptor should be introduced. All of the suggested methods have their pros and cons. Each of the proposed classifications tends to eliminate NSCLC subgroup heterogeneity while potentially being able to create new prognostic groups. Interestingly, only the LODDS is superior in terms of pN0 heterogeneity elimination (44). Cut-off values and potential prognostic groups vary from study to study, and they must be validated and determined in prospective international studies (e.g., IASLC Lung Cancer Staging Project). Additionally, in such a study, we could determine which of the descriptors is superior and should be introduced. Data regarding the NPLN and LNR are more robust (albeit retrospective) than other suggested N classifications, which need additional analyses. Hence, in our opinion, these 2 parameters or the LODDS (as the mathematical modification of the LNR) are more legitimate and should be considered first. Many more studies, especially regarding other classifications, need to be performed in the future. The minimal NDLN, in addition to being a good assessment of lymphadenectomy, is a factor that can be used to increase the objectivity of various proposed staging methods. The LODDS is potentially superior when minimal lymphadenectomy is not established. The NPLN is highly dependent on having a proper minimal NDLN. The LNR also benefits from more dissected lymph nodes, which was proven by Deng et al. (44).
It seems necessary that pN and cN descriptors should be divided (as in breast cancer), as quantity parameters of lymph nodes are not easily accessible in preoperative imaging and staging (7). For cN, ongoing TNM staging seems to be a good compromise for making treatment decisions. Additionally, as most of the data reviewed here are based on a population of resectable NSCLC patients (thus mostly pN), any changes regarding cN would not be appropriate. Even if a new nodal classification is introduced to the pathological staging of lung cancer, the nodal clinical staging would probably remain unchanged.
To summarize our review:
- Minimal lymphadenectomy in NSCLC should be settled;
- A quantity-based descriptor of lymph node metastases should be considered as an addition in the next TNM staging system;
- Prospective international validation study or studies need to be performed to validate optimal cut-off values and prognostic groups and to determine which newly suggested descriptor is superior.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at http://dx.doi.org/10.21037/jtd-20-1997
Peer Review File: Available at http://dx.doi.org/10.21037/jtd-20-1997
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd-20-1997). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019;69:7-34. [Crossref] [PubMed]
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Asamura H, Chansky K, Crowley J, et al. The International Association for the Study of Lung Cancer Lung Cancer Staging Project: Proposals for the Revision of the N Descriptors in the Forthcoming 8th Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2015;10:1675-84.
- Rusch VW, Crowley J, Giroux DJ, et al. The IASLC Lung Cancer Staging Project: Proposals for the Revision of the N Descriptors in the Forthcoming Seventh Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2007;2:603-12.
- Garelli E, Renaud S, Falcoz PE, et al. Microscopic N2 disease exhibits a better prognosis in resected non-small-cell lung cancer. Eur J Cardiothorac Surg 2016;50:322-8. [Crossref] [PubMed]
- Bille A, Woo KM, Ahmad U, et al. Incidence of occult pN2 disease following resection and mediastinal lymph node dissection in clinical stage I lung cancer patients. Eur J Cardiothorac Surg 2017;51:674-9. [Crossref] [PubMed]
- Brierley J, Gospodarowicz MK, Wittekind C. TNM classification of malignant tumours, 8th edition. Oxford, UK; Hoboken, NJ: John Wiley & Sons, Inc., 2017.
- Fukui T, Mori S, Yokoi K, et al. Significance of the number of positive lymph nodes in resected non-small cell lung cancer. J Thorac Oncol 2006;1:120-5. [Crossref] [PubMed]
- Lee JG, Lee CY, Park IK, et al. Number of metastatic lymph nodes in resected non-small cell lung cancer predicts patient survival. Ann Thorac Surg 2008;85:211-5. [Crossref] [PubMed]
- Saji H, Tsuboi M, Shimada Y, et al. A proposal for combination of total number and anatomical location of involved lymph nodes for nodal classification in non-small cell lung cancer. Chest 2013;143:1618-25. [Crossref] [PubMed]
- Lee GD, Kim DK, Moon DH, et al. A comparison of the proposed classifications for the revision of N descriptors for non-small-cell lung cancer. Eur J Cardiothorac Surg 2016;49:580-8. [Crossref] [PubMed]
- Hanagiri T, Takenaka M, Oka S, et al. Clinical significance in the number of involved lymph nodes in patients that underwent surgery for pathological stage III-N2 non-small cell lung cancer. J Cardiothorac Surg 2011;6:144. [Crossref] [PubMed]
- Wei S, Asamura H, Kawachi R, et al. Which is the better prognostic factor for resected non-small cell lung cancer: the number of metastatic lymph nodes or the currently used nodal stage classification? J Thorac Oncol 2011;6:310-8. [Crossref] [PubMed]
- Matsuguma H, Oki I, Nakahara R, et al. Proposal of new nodal classifications for non-small-cell lung cancer based on the number and ratio of metastatic lymph nodes. Eur J Cardiothorac Surg 2012;41:19-24. [PubMed]
- Fan Y, Du Y, Sun W, et al. Including positive lymph node count in the AJCC N staging may be a better predictor of the prognosis of NSCLC patients, especially stage III patients: a large population-based study. Int J Clin Oncol 2019;24:1359-66. [Crossref] [PubMed]
- Saji H, Tsuboi M, Yoshida K, et al. Prognostic impact of number of resected and involved lymph nodes at complete resection on survival in non-small cell lung cancer. J Thorac Oncol 2011;6:1865-71. [Crossref] [PubMed]
- Ma M, Tang P, Jiang H, et al. Number of negative lymph nodes as a prognostic factor in esophageal squamous cell carcinoma. Asia Pac J Clin Oncol 2017;13:e278-83. [Crossref] [PubMed]
- Liu H, Yan T, Zhang T, et al. Proposal of a new nodal classification for operable non-small cell lung cancer based on the number of negative lymph nodes and the anatomical location of metastatic lymph nodes. Medicine (Baltimore) 2019;98:e15645. [Crossref] [PubMed]
- Wang S, Zhang B, Li C, et al. Prognostic value of number of negative lymph node in patients with stage II and IIIa non-small cell lung cancer. Oncotarget 2017;8:79387-96. [Crossref] [PubMed]
- Doddoli C, Aragon A, Barlesi F, et al. Does the extent of lymph node dissection influence outcome in patients with stage I non-small-cell lung cancer? Eur J Cardiothorac Surg 2005;27:680-5. [Crossref] [PubMed]
- Ludwig MS, Goodman M, Miller DL, et al. Postoperative survival and the number of lymph nodes sampled during resection of node-negative non-small cell lung cancer. Chest 2005;128:1545-50. [Crossref] [PubMed]
- Yang M, Cao H, Guo X, et al. The number of resected lymph nodes (nLNs) combined with tumor size as a prognostic factor in patients with pathologic N0 and Nx non-small cell lung cancer. PLoS One 2013;8:e73220. [Crossref] [PubMed]
- Osarogiagbon RU, Ogbata O, Yu X. Number of lymph nodes associated with maximal reduction of long-term mortality risk in pathologic node-negative non-small cell lung cancer. Ann Thorac Surg 2014;97:385-93. [Crossref] [PubMed]
- Becker DJ, Levy BP, Gold HT, et al. Influence of Extent of Lymph Node Evaluation on Survival for Pathologically Lymph Node Negative Non-Small Cell Lung Cancer. Am J Clin Oncol 2018;41:820-5. [Crossref] [PubMed]
- Bria E, Milella M, Sperduti I, et al. A novel clinical prognostic score incorporating the number of resected lymph-nodes to predict recurrence and survival in non-small-cell lung cancer. Lung cancer 2009;66:365-71. [Crossref] [PubMed]
- Dai Y, Long H, Lin P, et al. Impact of the number of resected and involved lymph nodes on the outcome in patients with stage II non-small cell lung cancer. Zhonghua Zhong Liu Za Zhi 2010;32:436-40. [PubMed]
- Liang W, He J, Shen Y, et al. Impact of Examined Lymph Node Count on Precise Staging and Long-Term Survival of Resected Non-Small-Cell Lung Cancer: A Population Study of the US SEER Database and a Chinese Multi-Institutional Registry. J Clin Oncol 2017;35:1162-70. [Crossref] [PubMed]
- Ou S-HI, Zell JA. Prognostic significance of the number of lymph nodes removed at lobectomy in stage IA non-small cell lung cancer. J Thorac Oncol 2008;3:880-6. [Crossref] [PubMed]
- Samayoa AX, Pezzi TA, Pezzi CM, et al. Rationale for a Minimum Number of Lymph Nodes Removed with Non-Small Cell Lung Cancer Resection: Correlating the Number of Nodes Removed with Survival in 98,970 Patients. Ann Surg Oncol 2016;23:1005-11. [Crossref] [PubMed]
- Shapiro M, Mhango G, Kates M, et al. Extent of lymph node resection does not increase perioperative morbidity and mortality after surgery for stage I lung cancer in the elderly. Eur J Surg Oncol 2012;38:516-22. [Crossref] [PubMed]
- Wen YS, Xi KX, Xi KX, et al. The number of resected lymph nodes is associated with the long-term survival outcome in patients with T2 N0 non-small cell lung cancer. Cancer Manag Res 2018;10:6869-77. [Crossref] [PubMed]
- Riquet M, Legras A, Mordant P, et al. Number of mediastinal lymph nodes in non-small cell lung cancer: a Gaussian curve, not a prognostic factor. Ann Thorac Surg 2014;98:224-31. [Crossref] [PubMed]
- Chen SB, Weng HR, Wang G, et al. Lymph node ratio-based staging system for esophageal squamous cell carcinoma. World J Gastroenterol 2015;21:7514-21. [Crossref] [PubMed]
- Zhou J, Lin Z, Lyu M, et al. Prognostic value of lymph node ratio in non-small-cell lung cancer: a meta-analysis. Jpn J Clin Oncol 2020;50:44-57. [Crossref] [PubMed]
- Taylor MD, LaPar DJ, Thomas CJ, et al. Lymph node ratio predicts recurrence and survival after R0 resection for non-small cell lung cancer. Ann Thorac Surg 2013;96:1163-70. [Crossref] [PubMed]
- Wang CL, Li Y, Yue DS, et al. Value of the metastatic lymph node ratio for predicting the prognosis of non-small-cell lung cancer patients. World J Surg 2012;36:455-62. [Crossref] [PubMed]
- Hsieh CP, Fu JY, Liu YH, et al. Prognostic factors in resectable pathological N2 disease of non-small cell lung cancer. Biomed J 2015;38:329-35. [Crossref] [PubMed]
- Urban D, Bar J, Solomon B, et al. Lymph node ratio may predict the benefit of postoperative radiotherapy in non-small-cell lung cancer. J Thorac Oncol 2013;8:940-6. [Crossref] [PubMed]
- Renaud S, Falcoz PE, Olland A, et al. Mediastinal downstaging after induction treatment is not a significant prognostic factor to select patients who would benefit from surgery: the clinical value of the lymph node ratio. Interact Cardiovasc Thorac Surg 2015;20:222-7. [Crossref] [PubMed]
- Ding X, Hui Z, Dai H, et al. A Proposal for Combination of Lymph Node Ratio and Anatomic Location of Involved Lymph Nodes for Nodal Classification in Non-Small Cell Lung Cancer. J Thorac Oncol 2016;11:1565-73. [Crossref] [PubMed]
- Qiu C, Dong W, Su B, et al. The prognostic value of ratio-based lymph node staging in resected non-small-cell lung cancer. J Thorac Oncol 2013;8:429-35. [Crossref] [PubMed]
- Ding X, Dai H, Hui Z, et al. Risk factors of brain metastases in completely resected pathological stage IIIA-N2 non-small cell lung cancer. Radiat Oncol 2012;7:119. [Crossref] [PubMed]
- Ohtaki Y, Shimizu K, Kaira K, et al. Risk factors associated with recurrence of surgically resected node-positive non-small cell lung cancer. Surg Today 2016;46:1196-208. [Crossref] [PubMed]
- Deng W, Xu T, Wang Y, et al. Log odds of positive lymph nodes may predict survival benefit in patients with node-positive non-small cell lung cancer. Lung cancer 2018;122:60-6. [Crossref] [PubMed]
- Yang M, Zhang H, Ma Z, et al. Log odds of positive lymph nodes is a novel prognostic indicator for advanced ESCC after surgical resection. J Thorac Dis 2017;9:1182-9. [Crossref] [PubMed]
- Lv P, Chen G, Zhang P. Log odds of positive lymph nodes are superior to other measures for evaluating the prognosis of non-small cell lung cancer. Thorac Cancer 2014;5:570-5. [Crossref] [PubMed]
- Zhao Y, Li G, Zheng D, et al. The prognostic value of lymph node ratio and log odds of positive lymph nodes in patients with lung adenocarcinoma. J Thorac Cardiovasc Surg 2017;153:702-9.e1. [Crossref] [PubMed]
- Dziedzic D, Piotr R, Langfort R, et al. Log odds of positive lymph nodes as a novel prognostic indicator in NSCLC staging. Surg Oncol 2017;26:80-5. [PubMed]
- Chen W, Zhang C, Wang G, et al. Feasibility of nodal classification for non-small cell lung cancer by merging current N categories with the number of involved lymph node stations. Thorac Cancer 2019;10:1533-43. [Crossref] [PubMed]
- Yun JK, Lee GD, Choi S, et al. Comparison between lymph node station- and zone-based classification for the future revision of node descriptors proposed by the International Association for the Study of Lung Cancer in surgically resected patients with non-small-cell lung cancer. Eur J Cardiothorac Surg 2019;56:849-57. [Crossref] [PubMed]
- Park BJ, Kim TH, Shin S, et al. Recommended Change in the N Descriptor Proposed by the International Association for the Study of Lung Cancer: A Validation Study. J Thorac Oncol 2019;14:1962-9. [Crossref] [PubMed]
- Bertoglio P, Ricciardi S, Ali G, et al. N2 lung cancer is not all the same: an analysis of different prognostic groups. Interact Cardiovasc Thorac Surg 2018;27:720-6. [Crossref] [PubMed]
- Sayar A, Turna A, Kilicgun A, et al. Prognostic significance of surgical-pathologic multiple-station N1 disease in non-small cell carcinoma of the lung. Eur J Cardiothorac Surg 2004;25:434-8. [Crossref] [PubMed]
- Keller SM, Vangel MG, Wagner H, et al. Prolonged survival in patients with resected non-small cell lung cancer and single-level N2 disease. J Thorac Cardiovasc Surg 2004;128:130-7. [Crossref] [PubMed]
- Sezen CB, Aksoy Y, Sonmezoglu Y, et al. Prognostic factors for survival in patients with completely resected pN2 non-small-cell lung cancer. Acta Chir Belg 2019. [Epub ahead of print]. [Crossref] [PubMed]
- Ichinose Y, Kato H, Koike T, et al. Completely resected stage IIIA non-small cell lung cancer: the significance of primary tumor location and N2 station. J Thorac Cardiovasc Surg 2001;122:803-8. [Crossref] [PubMed]
- Nakagiri T, Sawabata N, Funaki S, et al. Validation of pN2 sub-classifications in patients with pathological stage IIIA N2 non-small cell lung cancer. Interact Cardiovasc Thorac Surg 2011;12:733-8. [Crossref] [PubMed]
- Martini N, Burt ME, Bains MS, et al. Survival after resection of stage II non-small cell lung cancer. Ann Thorac Surg 1992;54:460-5; discussion 466. [Crossref] [PubMed]
- Misthos P, Sepsas E, Kokotsakis J, et al. The significance of one-station N2 disease in the prognosis of patients with nonsmall-cell lung cancer. Ann Thorac Surg 2008;86:1626-30. [Crossref] [PubMed]
- Ito M, Yamashita Y, Tsutani Y, et al. Classifications of n2 non-small-cell lung cancer based on the number and rate of metastatic mediastinal lymph nodes. Clin Lung Cancer 2013;14:651-7. [Crossref] [PubMed]
- Cao Q, Zhang B, Zhao L, et al. The impact of positive nodal chain ratio on individualized multimodality therapy in non-small-cell lung cancer. Tumour Biol 2015;36:4617-25. [Crossref] [PubMed]
- Zheng H, Wang LM, Bao F, et al. Re-appraisal of N2 disease by lymphatic drainage pattern for non-small-cell lung cancers: by terms of nodal stations, zones, chains, and a composite. Lung Cancer 2011;74:497-503. [Crossref] [PubMed]
- Baba T, Uramoto H, Kuwata T, et al. Survival impact of node zone classification in resected pathological N2 non-small cell lung cancer. Interact Cardiovasc Thorac Surg 2012;14:760-4. [Crossref] [PubMed]
- Lardinois D, Suter H, Hakki H, et al. Morbidity, survival, and site of recurrence after mediastinal lymph-node dissection versus systematic sampling after complete resection for non-small cell lung cancer. Ann Thorac Surg 2005;80:268-74; discussion 274-5. [Crossref] [PubMed]
- Shapiro M, Kadakia S, Lim J, et al. Lobe-specific mediastinal nodal dissection is sufficient during lobectomy by video-assisted thoracic surgery or thoracotomy for early-stage lung cancer. Chest 2013;144:1615-21. [Crossref] [PubMed]
- Satoh Y, Okumura S, Nakagawa K, et al. Postoperative ischemic change in bronchial stumps after primary lung cancer resection. Eur J Cardiothorac Surg 2006;30:172-6. [Crossref] [PubMed]


