Hypersensitivity reactions to carboplatin and cisplatin in non-small cell lung cancer
Introduction
Lung cancer is the leading cause of cancer death among men and women in the United States (1). During the past ten years, treatment for early-stage and locally advanced non-small cell lung cancer (NSCLC) has changed as the results of large clinical trials have demonstrated a survival benefit from the use of adjuvant chemotherapy, typically 4 cycles of chemotherapy containing a platinum agent, which are given after recovery from surgical resection (2-5). A recent meta-analysis estimated a 5.4% overall survival benefit at 5 years among NSCLC cancer patients receiving adjuvant chemotherapy (6). Due to this change in lung cancer treatment, patients may now receive more than one course of platinum chemotherapy in their lifetime: after the initial surgical resection as adjuvant chemotherapy, and then later in the event of recurrent or metastatic cancer. Platinum hypersensitivity has been well characterized in the ovarian cancer population, with those patients treated for relapsed disease, after prior exposure to platinum chemotherapy, being at particularly great risk for experiencing a reaction (7,8). Accordingly, doctors and nurses caring for cancer patients should also be aware of the potential for platinum hypersensitivity in the lung cancer population.
Case
Our case is a 74-year-old caucasian male with a past medical history of stage IIB NSCLC (adenocarcinoma) who was treated with lobectomy in April 2006. Surgery was followed by adjuvant chemotherapy with carboplatin (AUC5) and paclitaxel (175 mg/m2) given for 4 cycles, which he completed in August 2006. Additionally, the patient had a history of hypertension, and transitional cell carcinoma of the bladder that was removed via transurethral resection (TURBT), and then treated with intravesicular Bacillus Calmette-Guerin (BCG) infusions. His father died from an unknown type of leukemia while in his eighties. The patient smoked roughly one pack of cigarettes daily for 50 years, quitting in 2006.
In December 2008, the patient was found to have lung nodules on a surveillance computed tomography (CT) scan which were biopsy proven to represent recurrence of his lung cancer. He then began treatment with intravenous carboplatin (AUC 5), pemetrexed (500 mg/m2) and bevacizumab (15 mg/kg). During the second cycle of this chemotherapy regimen he received pemetrexed over 15 minutes, followed by a 1 hour infusion of bevacizumab, and then carboplatin. Twenty-five minutes into the carboplatin infusion (his sixth lifetime exposure to carboplatin), the patient described feeling warmth in his head, and this sensation was closely followed by chest pressure and rigors. He denied shortness of breath or pruritis. Vital signs at the time of symptom onset revealed a temperature of 36 degrees C, blood pressure 146/88 mm Hg, pulse 109 bpm, respiratory rate of 22, pulse oxygenation 98% on room air. Physical examination was notable for tachycardia, generalized flushing, and urticaria on his chest and back. The carboplatin infusion was stopped, and the patient received an infusion of normal saline as well as intravenous diphenhydramine (25 mg) and methylprednisolone (100 mg). His symptoms resolved without further intervention, and vital signs recorded 1 hour after intravenous steroid administration were stable with blood pressure 123/73 mmHg and pulse 69 bpm. Carboplatin was removed from further cycles of chemotherapy.
Just prior to the reaction, the patient’s laboratory data revealed a creatinine of 0.8 mg/dL (estimated GFR >60 mL/min) and total bilirubin of 0.7 mg/dL. His regular medication included folic acid 1mg po daily, valsartan 80 mg/hydrochlorthiazide 12.5 mg daily, prochlorperazine 10 mg po as needed for nausea, and dexamethasone 4 mg po, taken the evening before and morning of chemotherapy as premedication. The patient had no known drug allergies prior to the described reaction.
Four additional lung cancer patients at our institution experienced hypersensitivity to a platinum agent (Table 1). In these cases, patients received an initial course of four to six cycles of platinum-based doublet chemotherapy, then had an extended break from chemotherapy (7 to 32 months), and were retreated with a platinum-doublet upon disease relapse or progression. Hypersensitivity reactions were all documented during the 2nd cycle of their retreatment courses.
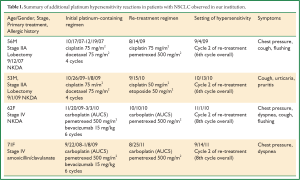
Full table
Discussion
Carboplatin and cisplatin have established themselves as efficacious chemotherapeutics, with utility in treating many solid tumors. Both carboplatin and cisplatin are platinum complexes, exerting their anticancer properties through formation of DNA adducts (9). Allergic responses in industrial workers exposed to platinum salts have been reported since 1945 (10) and allergic hypersensitivity reactions to platinum chemotherapeutics are widely reported, with much of the pertinent literature focused upon carboplatin hypersensitivity in setting of ovarian cancer treatment (11).
Hypersensitivity reactions to the platinum compounds are largely consistent with type 1, IgE mediated hypersensitivity, associated with repeated exposure to the agent. IgE is believed to act upon tissue mast cells and basophils in peripheral blood, prompting the release of histamines, leukotrienes and prostaglandins (12). These reactions are typified by capillary dilation and the rapid contraction of smooth muscle, often resulting in a rash (urticaria), flushing, and pruritis. In more severe cases (NCI CTCAE v4 Grade 3-4) angioedema, symptomatic bronchospasm, hypotension, and cardiac dysfunction may be evident, warranting immediate intervention (13). Symptoms usually develop during or within a few hours of drug infusion, but may occur 1-2 days after administration (7).
A ten-year study of carboplatin hypersensitivity in ovarian cancer patients reported an incidence rate of 16% (14), whereas a lower rate of 4.6% was found among patients at our institution between 2006 and 2010 (15). While estimates of the overall incidence of carboplatin hypersensitivity vary, it is important to understand that the cumulative incidence of carboplatin hypersensitivity increases considerably in proportion to the number of cycles administered (9). Patients receiving five of fewer cycles of carboplatin were reported to have a 0.92% incidence rate of hypersensitivity, with the cumulative risk increasing to 19.5% among patients who have received eight cycles (9). Markman, et al., noted that, in his study population, 50% of carboplatin hypersensitivity episodes occurred during course eight of platinum therapy, with some of the observed patients receiving cisplatin and then carboplatin in prior to the reaction (7).
Patients who receive first-line platinum-based chemotherapy, and then are treated again with a platinum agent at the time of relapse, are at the greatest risk of experiencing hypersensitivity. In a small study population of relapsed ovarian cancer patients, as many as 44% of patients who were given a second and third cycle of carboplatin, post-relapse, developed hypersensitivity (8). A more recent paper noted carboplatin hypersensitivity in 21.7% of patients treated for recurrent ovarian cancer. Of those who experienced a reaction, 86.7% did so during the second cycle of chemotherapy post-relapse, with prolonged retreatment interval (time elapsed between end of front-line therapy and beginning of carboplatin retreatment) having independent predictive value. Patients with a retreatment interval >23.4 months had an approximately seven-fold greater likelihood of developing a hypersensitivity reaction (16). Other studies have reported carboplatin-free intervals of >12 (17) or >13 months, as well as a cumulative carboplatin dose of >650 mg, as independent predictors of carboplatin hypersensitivity (18). The largest study of carboplatin hypersensitivity in the setting of recurrent ovarian cancer was published in 2011, involving over 900 patients. This study reports an overall hypersensitivity rate of 23.6% when carboplatin-based chemotherapy is re-introduced (19). Patients with a history of systemic allergic reactions to medication or environmental exposure also have a significantly increased risk of hypersensitivity (20).
Hypersensitivity reactions to cisplatin are also documented. An early study evaluating cisplatin in combination with vindesine as treatment for lung cancer described that, among patients receiving six or more cycles of combination chemotherapy, five of 21 patients experienced “anaphylaxis” characterized by urticaria, pruritis, respiratory distress, and mild hypotension within 10 minutes of the administration of cisplatin. In all cases the reaction subsided upon cessation of the cisplatin infusion and administration of epinephrine and diphenhydramine (21). Four cases of cisplatin hypersensitivity in ovarian cancer patients were later reported, two of which took place during the fifth administration of cisplatin. In another case the patient received three cycles of cisplatin following a debulking procedure, and experienced a reaction two years later when cisplatin treatment was resumed upon discovery of metastatic disease. A different patient, who received front-line carboplatin, relapsed after a six year treatment free interval and experienced cisplatin hypersensitivity on the third cycle of retreatment (22).
Intradermal skin testing has been used to predict hypersensitivity to carboplatin, with a very low false-negative rate of 1.5% (23).While a negative skin test may provide reassurance before administering carboplatin to at risk patients, the implications of a positive skin test are less clear. Changing to an alternative platinum agent following a reaction, e.g., switching from carboplatin to cisplatin after negative skin testing, has been reported (24). However, skin testing is not done at our institution and discontinuation of platinum chemotherapy entirely appears to be the most reasonable decision in the lung cancer patients who experience hypersensitivity. Platinum desensitization protocols, requiring inpatient hospitalization, are carried out for ovarian cancer patients who may benefit from continued platinum chemotherapy (25), however, such efforts would not typically be warranted in the setting of palliative chemotherapy for metastatic or recurrent lung cancer.
Three of our patients experienced a hypersensitivity reaction upon their sixth exposure to a platinum agent, with the last two patients experiencing a reaction on the eighth total exposure. In all cases, the patients received an initial course of four to six cycles of platinum-based doublet chemotherapy, then had an extended break from chemotherapy (7 to 32 months), and were retreated with a platinum-doublet upon disease progression. Hypersensitivity reactions all were documented during the second cycle of their retreatment courses, after a platinum-free interval.
The last two cases in the table did not receive adjuvant chemotherapy, but illustrate an analogous situation that may occur in the setting of patients who present with stage IV disease. These patients were treated initially with platinum-doublet chemotherapy, and were later re-challenged with a platinum agent after progressing through subsequent lines of non-platinum therapy. Such scenarios, with prolonged survival and preserved performance status in stage IV disease, allowing multiple opportunities for platinum treatment, should become more commonplace as lung cancer patients derive benefit from new targeted therapies. Lastly, although we have no cases to illustrate this patients with stage III disease who receive neoadjuvant chemotherapy, or concurrent chemotherapy and radiation containing a platinum agent, would in principle, carry a similar future risk of platinum hypersensitivity if treated with a platinum chemotherapeutic for relapsed disease. The overall incidence of platinum hypersensitivity among lung cancer patients at our institution has not yet been determined.
Conclusions
These cases illustrate that NSCLC patients may experience hypersensitivity reactions to both carboplatin and cisplatin, and such reactions occur in a pattern consistent with that described by the gynecologic oncology literature. Patients who have previously received multiple exposures to platinum chemotherapy are at risk of a reaction, and those patients who are beginning platinum-based chemotherapy for relapsed cancer (after a prior exposure to platinum chemotherapy and a treatment-free interval) are at great risk. All “at risk” lung cancer patients must be counseled extensively about the risk of platinum hypersensitivity, and be aware of the symptoms they should report to their treatment team. Clinicians and infusion center nursing staff should be aware of these risks and be able to respond urgently in the event of a reaction, and these patients should be scheduled for chemotherapy only during times where a physician is readily available.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin 2012;62:10-29.
- Arriagada R, Bergman B, Dunant A, et al. Cisplatin-based adjuvant chemotherapy in patients with completely resected non-small-cell lung cancer. N Engl J Med 2004;350:351-60.
- Winton T, Livingston R, Johnson D, et al. Vinorelbine plus cisplatin vs. observation in resected non-small-cell lung cancer. N Engl J Med 2005;352:2589-97.
- Douillard JY, Rosell R, De Lena M, et al. Adjuvant vinorelbine plus cisplatin versus observation in patients with completely resected stage IB-IIIA non-small-cell lung cancer (Adjuvant Navelbine International Trialist Association [ANITA]): a randomised controlled trial. Lancet Oncol 2006;7:719-27.
- Strauss GM, Herndon JE 2nd, Maddaus MA, et al. Adjuvant paclitaxel plus carboplatin compared with observation in stage IB non-small-cell lung cancer: CALGB 9633 with the Cancer and Leukemia Group B, Radiation Therapy Oncology Group, and North Central Cancer Treatment Group Study Groups. J Clin Oncol 2008;26:5043-51.
- Pignon JP, Tribodet H, Scagliotti GV, et al. Lung adjuvant cisplatin evaluation: a pooled analysis by the LACE Collaborative Group. J Clin Oncol 2008;26:3552-9.
- Markman M, Kennedy A, Webster K, et al. Clinical features of hypersensitivity reactions to carboplatin. J Clin Oncol 1999; 17:1141-5.
- Morgan JS, Adams M, Mason MD. Hypersensitivity reactions to carboplatin given to patients with relapsed ovarian carcinoma. Eur J Cancer 1994;30A:1205-6.
- Sliesoraitis S, Chikhale PJ. Carboplatin hypersensitivity. Int J Gynecol Cancer 2005;15:13-8.
- Cleare MJ, Hughes EG, Jacoby B, et al. Immediate (type I) allergic responses to platinum compounds. Clin Allergy 1976;6:183-95.
- Navo M, Kunthur A, Badell ML, et al. Evaluation of the incidence of carboplatin hypersensitivity reactions in cancer patients. Gynecol Oncol 2006;103:608-13.
- Lenz HJ. Management and preparedness for infusion and hypersensitivity reactions. Oncologist 2007;12:601-9.
- National Cancer Institute: Common Terminology Criteria for Adverse Events v4.03 (CTCAE). Publish date June 14, 2010. Available online: http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-_QuickReference_8.5x11.pdf (accessed 2012 Jan 31).
- Polyzos A, Tsavaris N, Kosmas C, et al. Hypersensitivity reactions to carboplatin administration are common but not always severe: a 10-year experience. Oncology 2001;61:129-33.
- DeMoor PA, Matusov Y, Kelly C, et al. A retrospective review of the frequency and nature of acute hypersensitivity reactions at a medium-sized infusion center: comparison to reported values and inconsistencies found in literature. J Cancer 2011;2:153-64.
- Gadducci A, Tana R, Teti G, et al. Analysis of the pattern of hypersensitivity reactions in patients receiving carboplatin retreatment for recurrent ovarian cancer. Int J Gynecol Cancer 2008;18:615-20.
- Schwartz JR, Bandera C, Bradley A, et al. Does the platinum-free interval predict the incidence or severity of hypersensitivity reactions to carboplatin? The experience from Women and Infants’ Hospital. Gynecol Oncol 2007;105:81-3.
- Sugimoto H, Iwamoto T, Murashima Y,et al. Risk factors contributing to the development of carboplatin-related delayed hypersensitivity reactions in Japanese patients with gynecologic cancers. Cancer Chemother Pharmacol 2011;67:415-19.
- Joly F, Ray-Coquard I, Fabbro M, et al. Decreased hypersensitivity reactions with carboplatin-pegylated liposomal doxorubicin compared to carboplatin-paclitaxel combination: analysis from the GCIG CALYPSO relapsing ovarian cancer trial. Gynecol Oncol 2011;122:226-32.
- Markman M, Zanotti K, Kulp B, et al. Relationship between a history of systemic allergic reactions and risk of subsequent carboplatin hypersensitivity. Gynecol Oncol 2003;89:514-6.
- Gralla RJ, Casper ES, Kelsen DP, et al. Cisplatin and vindesine combination chemotherapy for advanced carcinoma of the lung: A randomized trial investigating two dosage schedules. Ann Intern Med 1981;95:414-20.
- Saunders MP, Denton CP, O’Brien ME, et al. Hypersensitivity reactions to cisplatin and carboplatin--a report on six cases. Ann Oncol 1992;3:574-6.
- Markman M, Zanotti K, Peterson G, et al. Expanded experience with an intradermal skin test to predict for the presence or absence of carboplatin hypersensitivity. J Clin Oncol 2003;21:4611-4.
- Syrigou E, Makrilia N, Vassias A, et al. Administration of cisplatin in three patients with carboplatin hypersensitivity: is skin testing useful? Anti-Cancer Drugs 2010;21:333-8.
- Hesterberg PE, Banerji A, Oren E, et al. Risk stratification for desensitization of patients with carboplatin hypersensitivity: clinical presentation and management. J Allergy Clin Immunol 2009;123:1262-7.e1.


