Predictive factors related to pleural dissemination in non-small cell lung cancer
Introduction
Lung cancer is the leading cause of cancer-related mortality worldwide (1). In the 8th edition of the American Joint Committee on Cancer (AJCC)/Union for International Cancer Control (UICC) staging system of non-small cell lung cancer (NSCLC) based on tumor, nodal and metastatic (TNM) staging system, pleural dissemination was categorized as M1a (2). The prognosis of NSCLC patients with M1a is poor, with a median survival time of 11.5 months and a 5-year survival rate of 10%; therefore (3), pleural dissemination is generally considered a contraindication for radical surgery (4-6).
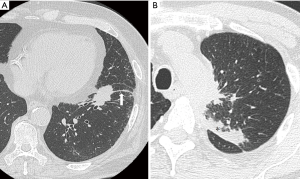
Solid pleural metastasis without malignant pleural effusion is defined as dry pleural dissemination (DPD) (7,8). While the utility of computed tomography (CT), positron emission tomography (PET), and radiomics for preoperatively detecting DPD has been reported (9-11), small localized pleural dissemination might be detected or missed intraoperatively in patients with clinical stage M0 (Figure 1). If pleural dissemination is detected intraoperatively, patients can receive appropriate chemotherapy as stage IV NSCLC. Furthermore, because of the prognostic improvement of curative surgery followed by chemotherapy for NSCLC patients with localized pleural dissemination (12-18), surgery might be an option in multidisciplinary treatment for stage IV NSCLC patients. However, if pleural dissemination is missed intraoperatively, patients with false-negative stage IV NSCLC cannot receive appropriate chemotherapy, and their prognosis might worsen. We hypothesized that if pleural dissemination is predicted by clinicopathological factors preoperatively, patients can receive appropriate chemotherapy as stage IV NSCLC.
In the present study, we retrospectively analyzed the relationship between clinicopathological factors and pleural dissemination in NSCLC patients and examined whether or not it was possible to assess the risk of DPD before surgery.
We present the following article in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting checklist (available at http://dx.doi.org/10.21037/jtd-20-1543).
Methods
Study patients
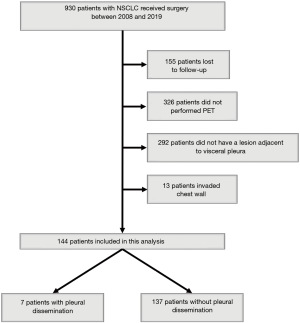
Nine hundred and thirty NSCLC patients who underwent surgery in Kanazawa Medical University between January 2008 and December 2019 were identified. Among these, 155 patients were lost to follow-up, 326 did not undergo PET, 292 did not have a lesion adjacent to the visceral pleura, and 13 had lesions invading the chest wall. Therefore, 144 patients were the subject of the present retrospective analysis (Figure 2). The lifetime cigarette consumption was assessed using the Brinkman index, which is calculated as the number of cigarettes smoked per day multiplied by the number of years for which the subject has smoked.
Tumor diameter
The maximum tumor diameter and diameter of tumors adjacent to the visceral pleura were measured on chest CT by two radiologists. The maximum tumor diameter on CT was defined as the CTmax, and the diameter of tumors adjacent to the visceral pleura was defined as the Pmax. The maximum tumor diameter on a pathological examination was defined as the Pathomax.
Maximum standardized uptake value (SUVmax)
18F-fluoro-2-deoxy-glucose (18F-FDG)-PET was performed with a dedicated PET camera (SIEMENS Biograph Sensation 16; SIEMENS, Erlangen Germany) before surgery. All patients fasted for 6 h before scanning. The dose of 18F-FDG administered was 3.7 MBq/kg of the patient’s body weight. After a 60-min uptake period, an emission scan was acquired for 3 min per bed position, and a whole-body scan was performed according to the height of each patient. After image reconstruction, a two-dimensional (2D) round region of interest (ROI) was drawn on a slice after visual detection of the highest count on the fused CT image. For lesions with negative or faintly positive PET findings, the ROI was drawn on the fusion image obtained with the corresponding CT image. From those ROIs, the SUVmax was calculated by two radiologists.
Blood chemistry test
The preoperative white blood cell (WBC) count, platelet (Plt) count, lactate dehydrogenase (LDH), and C-reactive protein (CRP) were analyzed as predictive factors of pleural dissemination. Furthermore, the preoperative neutrophil-to-lymphocyte ratio (NLR) was analyzed as well.
Clinical and pathological diagnoses
Clinical and pathological TNM staging was performed in all patients based on the eighth edition of the AJCC/UICC classification. According the TNM staging system, pleural dissemination was classified as M1a.
Statistical analyses
Pearson’s chi-square test for independence was used to compare frequencies of clinicopathologic variables. The cumulative survival rates were calculated by the Kaplan-Meier methods, and survival curves were compared using the log-rank test. The cut-off value for the clinicopathological factors related to dissemination was calculated according to a receiver operating characteristics (ROC) curve analysis. The predictive factors related to dissemination were analyzed by a logistic regression analysis. All statistical analyses were two-sided, and statistical significance was defined as a P value of less than 0.05. The statistical analyses were conducted using the JMP software program (Version 13.2; SAS Institute Inc., Cary, NC, USA).
Ethical statement
The institutional review boards of Kanazawa Medical University approved the protocol (the approval number: I449), and written informed consent was obtained from all patients. The study conformed to the provisions of the Declaration of Helsinki (as revised in 2013).
Results
Patients’ characteristics
The clinicopathologic characteristics of these 144 patients are listed in Table 1. One hundred and ten patients were men, and the median age of patients was 68 years old. The median smoking index was 822, the median carcinoembryonic antigen (CEA) was 5.2 ng/mL, and the median SUVmax was 9.40. The median CTmax was 40 mm, and the median Pmax was 29 mm. The median WBC was 6,380/m2, the median Plt was 248,000/m2, the median NLR was 2.39, the median LDH was 193 U/L, and the median CRP was 0.19 mg/dL. Seven patients had pleural dissemination detected intraoperatively. The histopathological type was adenocarcinoma in 73 patients, squamous cell carcinoma in 53 patients, and other types in 18 patients. The histological type of patients with pleural dissemination was adenocarcinoma in 6 patients and large cell neuroendocrine carcinoma in 1 patient. The median Pathomax was 42 mm, and the histological grade of differentiation (G) was G1 (well differentiated) in 35 patients, G2 (moderately differentiated) in 61 patients, G3 (poorly differentiated) in 38 patients, and G4 (undifferentiated) in 10 patients. The histological grade of differentiation of patients with pleural dissemination was G3 in 6 patients and G4 in 1 patient. 3-year overall survival was not significant difference between patients with pleural dissemination and without pleural dissemination (100% vs. 77.6%, P=0.238).

Full table
Relationship between pleural dissemination and clinicopathological variables
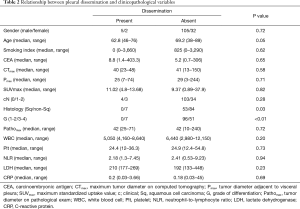
The relationship between pleural dissemination and clinicopathological variables are shown in Table 2. There were significant differences in the histopathological type (P=0.03), and differentiation (P<0.01).
Full table
Analysis of non-squamous cell carcinoma
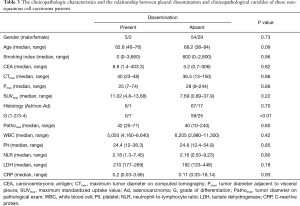
Because squamous cell carcinoma was considered to tend not to show dissemination to the pleural cavity in the early term, the predictive factors related to pleural dissemination in non-squamous cell carcinoma patients were analyzed. The clinicopathological characteristics and the relationship between pleural dissemination and the clinicopathological variables of these non-squamous cell carcinoma patients are shown in Table 3. A significant difference in the differentiation was noted (P<0.01).
Full table
The cut-off value for the clinicopathological variables related to pleural dissemination was calculated by a ROC curve analysis, and the relationships between pleural dissemination and the clinicopathological variables of these non-squamous cell carcinoma patients are shown in Table 4. There were significant differences in the age (P=0.02), smoking index (P=0.03), SUVmax (P=0.03), and differentiation (P<0.01).
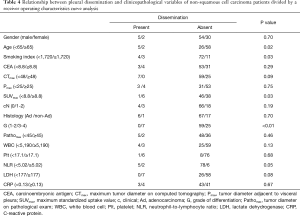
Full table
Logistic regression analyses
The results of logistic regression analyses of the predictive factors related to pleural dissemination are summarized in Table 5. Young age (P=0.01) and G3 or G4 (P<0.01) were identified as significant predictive factors related to pleural dissemination.
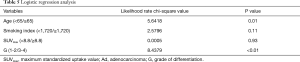
Full table
Discussion
Pleural dissemination has generally been reported to show a poor prognosis in patients with NSCLC and is a contraindication for radical surgery (2-6). In the 8th edition of the AJCC/UICC staging system of NSCLC based on TNM staging system, pleural dissemination was categorized in M1a (2). However, detecting localized small pleural dissemination without pleural effusion preoperatively is considered difficult. Therefore, identifying the predictive factors related to pleural dissemination would be valuable for ensuring appropriate therapy is administered to patients with stage IV NSCLC.
In the present study, several factors related to pleural dissemination were identified. First, squamous cell carcinoma tends not to disseminate to the pleural cavity compared to non-squamous cell carcinoma. Although the rate of pleural dissemination of squamous cell carcinoma was reported to be almost 20% in patients with pleural effusion (19), the rate of pleural dissemination of squamous cell carcinoma was reported to be 8% in patients without pleural effusion (20). Squamous cell carcinoma might tend not to disseminate to the pleural cavity in the early term. Second, young patients with non-squamous cell carcinoma tended to develop pleural dissemination more frequently than older patients in the present study. Although the incident rate of pleural dissemination in NSCLC was reported to be 1–1.7% in a population-based study (21,22), it was reported in another study that approximately 40% of patients ≤35 years of age with lung cancer developed pleural dissemination (23). While the prognosis of elderly patients with NSCLC is reportedly worse than that of younger patients (24), it cannot be deny that young patients with NSCLC develop pleural dissemination earlier than elderly patients. Third, histological differentiation of G3 and 4 was a predictive factor related to pleural dissemination in patients with non-squamous cell carcinoma in the present study. As the histological grade was reported to be an independent prognostic factor of patients with NSCLC in previous studies (25,26), poorly differentiated or undifferentiated non-squamous cell carcinoma might tend to disseminate to the pleural cavity. In young patients with NSCLC suspected of having non-squamous cell carcinoma or poorly differentiated and undifferentiated preoperatively, surgery should be performed as soon as possible, before dissemination to the pleural cavity can occur.
Although the pleural dissemination associated to NSCLC is categorized as M1a in the 8th edition of the TNM staging system and generally considered a contraindication for surgery, surgical intervention for NSCLC patients with pleural dissemination reportedly improved the prognosis in several studies (13-19,22,27-32). While the 5-year survival rate of NSCLC patients with M1a is 10% (3), the 5-year survival rate of NSCLC patients with pleural dissemination who received surgical intervention was reported to be 13.1–42.7% (13-19,22,27-32). Although surgical intervention for NSCLC patients with pleural dissemination might improve the prognosis, the recommended surgical procedure is unclear. While complete surgical resection of NSCLC associated with limited metastatic pleural dissemination was reported to be associated with a long-term survival (18), several studies have found no significant differences in the outcomes between radical resection (pneumonectomy, bilobectomy, and lobectomy) and limited resection (segmentectomy and partial resection) (15-17). Furthermore, it was reported that resection of the main tumor and pleural nodule in patients with lung adenocarcinoma with intraoperatively diagnosed pleural dissemination improved the prognosis (27). In those reports, main tumor resection was considered debulking surgery as part of the multidisciplinary treatment of NSCLC patients with pleural dissemination. Although the patients with pleural dissemination did not receive debulking surgery in our study, overall survival was not significant difference between patients with pleural dissemination and without pleural dissemination. It is considered that the survival for NSCLC patients is improving due to the progress of chemotherapy, and then survival might improve by main tumor resection as part of the multidisciplinary treatment.
Limitations
This study is associated with some limitations, including the small sample size, retrospective nature, and single-institution setting. Furthermore, there were only 7 patients with pleural dissemination for 11-year, it is possible to dismiss the patients with pleural dissemination. The appropriate operative procedure for patients with DPD is unclear at this time, and then it is considered important to elucidate the appropriate operative procedure for DPD, in the future.
In conclusions, three predictive factors were suggested to be related to pleural dissemination due to NSCLC in the present study. Cases with non-squamous cell carcinoma, a young age, and poor differentiation of undifferentiated grade of histological differentiation might tend to disseminate to pleural cavity in early time. Surgery should therefore be performed as soon as possible when these predictive factors are suspected, before dissemination to the pleural cavity can occur.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/jtd-20-1543
Data Sharing Statement: Available at http://dx.doi.org/10.21037/jtd-20-1543
Peer Review File: Available at http://dx.doi.org/10.21037/jtd-20-1543
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd-20-1543). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The institutional review boards of Kanazawa Medical University approved the protocol (the approval number: I449), and written informed consent was obtained from all patients. The study conformed to the provisions of the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Eberhardt WE, Mitchell A, Crowley J, et al. The IASLC lung cancer staging project: proposals for the revision of the M descriptors in the forthcoming eighth edition of the TNM classification of lung cancer. J Thorac Oncol 2015;10:1515-22.
- Goldstraw P, Chansky K, Crowley J, et al. The IASLC lung cancer staging project: proposals for revision of the TNM stage groupings in the forthcoming (eighth) edition of the TNM classification for lung cancer. J Thorac Oncol 2016;11:39-51. [Crossref] [PubMed]
- American Joint Committee on Cancer. Lung, In: Rami-Porta R, Asamura H, Travis WD, Rusch VW, eds. AJCC Cancer Staging Manual. 8th ed. New York: Springer; 2017:431-456.
- Sugiura S, Ando Y, Minami H, et al. Prognostic value of pleural effusion in patients with non-small cell lung cancer. Clin Cancer Res 1997;3:47-50. [PubMed]
- Jett JR, Scott WJ, Rivera MP, et al. Guidelines on treatment of Stage IIIB non-small cell lung cancer. Chest 2003;123:221S-225S. [Crossref] [PubMed]
- Johnston WW. The malignant pleural effusion. A review of cytopathologic diagnoses of 584 specimens from 472 consecutive patients. Cancer 1985;56:905-9. [Crossref] [PubMed]
- Shim SS, Lee KS, Kim BT, et al. Integrated PET/CT and the dry pleural dissemination of peripheral adenocarcinoma of the lung: diagnostic implications. J Comput Assist Tomogr 2006;30:70-6. [Crossref] [PubMed]
- Choe DH, Sohn JE, Lee TH, et al. CT findings of pleural dissemination from lung cancer. J Korean Radiol Soc 1999;41:1139-45. [Crossref]
- Okutani D, Yamane M, Toyooka S, et al. Dry small pleural dissemination of adenocarcinoma of the lung preoperatively detected by PET/CT: a report of two cases. Acta Med Okayama 2008;62:55-8. [PubMed]
- Yang M, Ren Y, She Y, et al. Imaging phenotype using radiomics to predict dry pleural dissemination in non-small cell lung cancer. Ann Transl Med 2019;7:259. [Crossref] [PubMed]
- Chikaishi Y, Shinohara S, Kuwata T, et al. Complete resection of the primary lesion improves survival of certain patients with stage IV non-small cell lung cancer. J Thorac Dis 2017;9:5278-87. [Crossref] [PubMed]
- Ren YJ, She YL, Dai CY, et al. Primary tumour resection showed survival benefits for non-small-cell lung cancers with unexpected malignant pleural dissemination. Interact Cardiovasc Thorac Surg 2016;22:321-6. [Crossref] [PubMed]
- Yun JK, Kim MA, Choi CM, et al. Surgical Outcomes after Pulmonary Resection for Non-Small Cell Lung Cancer with Localized Pleural Seeding First Detected during Surgery. Thorac Cardiovasc Surg 2018;66:142-9. [Crossref] [PubMed]
- Liu T, Liu H, Wang G, et al. Survival of M1a Non-Small Cell Lung Cancer Treated Surgically: A Retrospective Single-Center Study. Thorac Cardiovasc Surg 2015;63:577-82. [Crossref] [PubMed]
- Go T, Misaki N, Matsuura N, et al. Role of surgery in multi-modality treatment for carcinomatous pleuritis in patients with non-small cell lung cancer. Surg Today 2015;45:197-202. [Crossref] [PubMed]
- Okamoto T, Iwata T, Mizobuchi T, et al. Pulmonary resection for lung cancer with malignant pleural disease first detected at thoracotomy. Eur J Cardiothorac Surg 2012;41:25-30. [PubMed]
- Mordant P, Arame A, Foucault C, et al. Surgery for metastatic pleural extension of non-small-cell lung cancer. Eur J Cardiothorac Surg 2011;40:1444-9. [Crossref] [PubMed]
- Ren Y, Dai C, Shen J, et al. The prognosis after contraindicated surgery of NSCLC patients with malignant pleural effusion (M1a) may be better than expected. Oncotarget 2016;7:26856-65. [Crossref] [PubMed]
- Shiono S, Nagai K, Nishimura M, et al. Pleural dissemination predictors based on preoperative and radiological findings in lung cancer patients. JJLC 2003;43:687-90. [Crossref]
- Committee for Scientific Affairs, The Japanese Association for Thoracic Surgery, Shimizu H, Endo S, et al. Thoracic and cardiovascular surgery in Japan in 2016. Gen Thorac Cardiovasc Surg 2019;67:377-411. [Crossref] [PubMed]
- Li H, Sun Z, Yang F, et al. Primary tumour resection in-non-small-cell lung cancer patients with ipsilateral pleural dissemination (M1a): a population-based study. Eur J Cardiothorac Surg 2019;55:1121-9. [Crossref] [PubMed]
- Liu B, Quan X, Xu C, et al. Lung cancer in young adults aged 35 years or younger: a full-scale analysis and review. J Cancer 2019;10:3553-9. [Crossref] [PubMed]
- Tas F, Ciftci R, Kilic L, et al. Age is a prognostic factor affecting survival in lung cancer patients. Oncol Lett 2013;6:1507-13. [Crossref] [PubMed]
- Barletta JA, Yeap BY, Chirieac JR. The prognostic significance of grading in lung adenocarcinoma. Cancer 2010;116:659-69. [Crossref] [PubMed]
- Yasukawa M, Sawabata N, Kawaguchi T, et al. Histological grade: analysis of prognosis of non-small cell lung cancer after complete resection. In Vivo 2018;32:1505-12. [Crossref] [PubMed]
- Li C, Kuo SW, Hsu HH, et al. Lung adenocarcinoma with intraoperatively diagnosed pleural seeding: Is main tumor resection beneficial for prognosis? J Thorac Cardiovasc Surg 2018;155:1238-49.e1. [Crossref] [PubMed]
- Iida T, Shiba M, Yoshino I, et al. Surgical intervention for non-small-cell lung cancer patients with pleural carcinomatosis. Result from the Japanese Lung Cancer Registry in 2004. J Thorac Oncol 2015;10:1076-82. [Crossref] [PubMed]
- Ohta Y, Tanaka Y, Hara T, et al. Clinicopathological and biological assessment of lung cancers with pleural dissemination. Ann Thorac Surg 2000;69:1025-9. [Crossref] [PubMed]
- Ichinose Y, Tsuchiya R, Koike T, et al. The prognosis of patients with non-small cell lung cancer found to have carcinomatous pleuritis at thoracotomy. Surg Today 2000;30:1062-6. [Crossref] [PubMed]
- Shiba M, Kakizawa K, Kohno H, et al. Prognostic implication of Ki-67 immunostaining in treating subclinical pleural cancer found at thoracotomy in lung cancer patients. Ann Thorac Surg 2001;71:1765-71. [Crossref] [PubMed]
- Yokoi K, Matuguma H, Anraku M. Extrapleural pneumonectomy for lung cancer with carcinomatous pleuritis. J Thorac Cardiovasc Surg 2002;123:184-5. [Crossref] [PubMed]