
The influence of thoracic duct ligation on long-term survival of patients with esophageal cancer: a propensity score-matched analysis
Introduction
The thoracic duct (TD) is part of the lymphatic system and functions to carry chyle. Chyle is a fluid which contains emulsified fats, proteins, sugars, lymphocytes, immunoglobulins, and various enzymes (1). The TD therefore plays a fundamental role in maintaining homeostasis. It is also prone to sustaining damage during thoracic operations for malignancies such as esophagectomy and lobectomy (2,3). Postoperative chylothorax is a rare but serious complication that can occur after esophagectomy, with a reported incidence of up to 10% (4). If left untreated, chylothorax can lead to malnutrition, infection, circulatory failure, and even death (5). Since Lampson reported the first successful case in 1948 (6), ligation of the thoracic duct (LTD) has been used to prevent postoperative chylothorax after esophagectomy.
Previous studies have focused on the reduction of chylothorax as the only clinical goal in LTD, thus overlooking its impact on overall long-term survival (7). In a retrospective study, Hou et al. showed that prophylactic LTD had an unfavorable impact on the overall survival (OS) of patients with esophageal cancer (8). However, there were many confounders in the treatment groups, which may have introduced selection bias and reduced the credibility of the results. Therefore, the impact of LTD on long-term survival warrants further investigation.
To this end, we conducted a retrospective analysis using propensity score matching (PSM) to control for confounding factors between the two groups, and assessed whether LTD could affect long-term survival rates. We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/jtd-20-1341).
Methods
Study population
A retrospective analysis of 634 consecutive patients with esophageal carcinoma who underwent an esophagectomy between September, 2012, and January, 2014, at Changhai Hospital was conducted. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Ethics Committee of Changhai Hospital (CHEC-2019-359) and informed consent was taken from all the patients. All patients included in the analysis fit the following criteria: (I) pathologically confirmed esophageal carcinoma or esophagogastric junction carcinoma; (II) tumors were completely resected during esophagectomy (R0 resection); and (III) patients had complete medical records. The exclusion criteria were as follows: (I) a history of previous or concurrent malignancies; or (II) incomplete resection of tumors.
Of the 634 esophageal carcinoma patients who underwent esophagectomy at Changhai Hospital, 609 were included in the analysis. Of the 25 patients excluded from the analysis, 18 had concurrent or previous malignancies and 7 had undergone incomplete resection. All of the patients included in the study were re-staged according to the 8th edition of the American Joint Committee on Cancer (AJCC) classification system. Patients were classified into two groups based on their medical records: the thoracic duct ligation group (LG, n=241) and the non-ligation group (NLG, n=368).
Surgical procedures and postoperative management
Radical esophagectomy and regional lymph node dissections were performed in all patients. For the LG, the thoracic duct was ligated 2 cm above the diaphragm, approximately at the level of the ninth thoracic vertebra. The thoracic duct was ligated when intraoperative chylous leakage occurred, or injury to the thoracic duct was highly suspected.
Postoperative complications were defined as the occurrence of any surgery-related complications taking place in hospital after surgery, including chylothorax, anastomotic leak, recurrent nerve palsy, and pneumonia. Postoperative chylothorax was defined as triglyceride levels >110 mL/day or by the presence of chylomicrons found in pleural fluid. Conservative treatment or LTD was applied to treat chylothorax according to each patient’s condition. Other complications were diagnosed using the relevant diagnostic criteria.
Follow-up
Follow-up data were collected by contacting patients and their relatives by telephone or by obtaining their medical records. Routine examinations, such as physical examinations, blood chemistry analysis, and computed tomography (CT) scans of the thorax and abdomen were generally performed every 3 months for the first 2 years and every 6 months after that for 5 years. After 5 years, the patients were assessed annually. The end of the follow-up period was January, 2019. The median follow-up time was 46 months (range, 1–81 months) for all patients, and the rate of patients lost to follow-up was 13.3%. Patients lost to follow-up were treated as censored data. All-cause mortality was the primary endpoint of this study.
Statistical analysis
Statistical analyses were performed using SPSS 24.0 (IBM, Chicago, IL, USA). Continuous variables were reported as mean ± standard deviation (SD) and compared between the groups using t-tests. Categorical variables were reported as proportions and analyzed using a chi-square test. To minimize the differences between the two groups (LG vs. NLG), 1:1 propensity score matching (PSM) was performed, with a caliper width of 0.1. Propensity scores were based on age, gender, BMI, hypertension, diabetes, smoking, drinking, tumor location, surgical approach, pathologic T stage (pT stage), pathologic N stage (pN stage), pathological type, tumor cell differentiation, minimally invasive surgery (MIS), neoadjuvant therapy, and endoscopic submucosal dissection (ESD). Survival rates were estimated using the Kaplan-Meier method and the differences between the two groups were analyzed using the log-rank test. Independent risk factors were determined using univariate and multivariate cox regression analyses. A P value of less than 0.05 was considered to be statistically significant.
Results
Patient characteristics
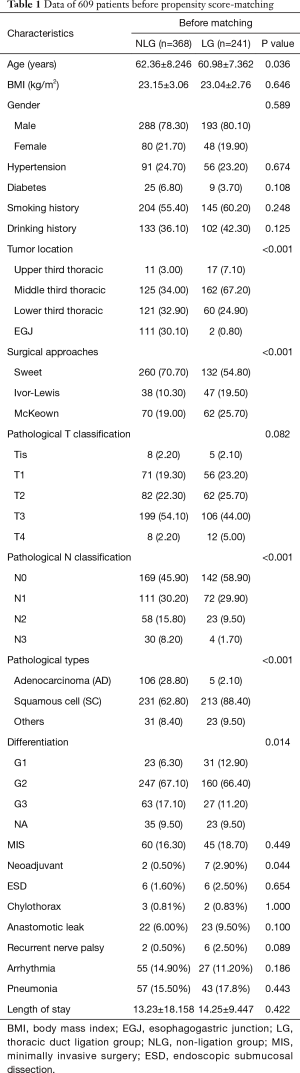
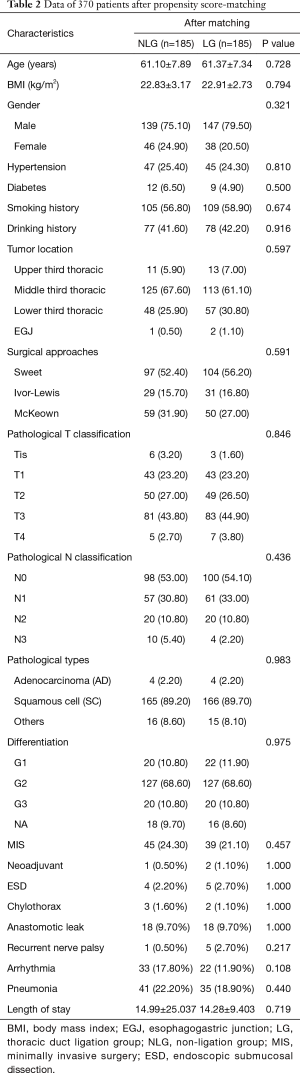
Tables 1 and 2 show the baseline data of all the patients. Before matching, a total of 241 (39.57%) and 368 (60.43%) patients were assigned to the LG and NLG, respectively. The patients in the LG were significantly younger than those of NLG (60.98±7.362 vs. 62.36±8.246; P=0.036). NLG had more patients with EGJ tumors (30.10% vs. 0.80%; P<0.001) and adenocarcinomas (28.80% vs. 2.10%; P<0.001), while the LG included more patients with pN0 stage (58.90% vs. 45.90%; P<0.001) and neoadjuvant therapy (2.9% vs. 0.5%; P=0.044). A total of 260 (70.70%) patients in the NLG received the Sweet procedure, compared with 54.80% of patients in the LG (P<0.001). Furthermore, tumor differentiation in the LG was better than that in the NLG (G1:12.90% vs. 6.30%; P=0.014). There were no significant differences in BMI, gender, comorbidities, pT stage, smoking and drinking history, MIS, or ESD between the two groups. After PSM, the cohorts were narrowed to 185 patients in each group. All of the baseline data were comparable between the two groups.
Full table
Full table
Postoperative complications
Postoperative chylothorax occurred in 2 patients in the LG (0.83%) and 3 patients in the NLG (0.81%), with no significant difference (P=1.000). Except for reoperation in 1 patient in the LG, the 4 other patients were treated with conservative approaches, and there were no hospital mortalities among these patients. No significant difference was found between the two groups for incidence of anastomotic leak, recurrent nerve palsy, arrhythmia, or pneumonia, nor was a significant difference found in length of hospital stay, as shown in Table 1. After PSM, chylothorax incidence in the LG was lower than that of the NLG (1.1% vs. 1.6%), although this difference was not significant (P=1.000). LTD did not increase the risk of anastomotic leak (9.7% vs. 9.7%; P=1.000), recurrent nerve palsy (2.7% vs. 0.5%; P=0.217), arrhythmia (11.90% vs. 17.80%; P=0.108), pneumonia (18.9% vs. 22.2%; P=0.440), or length of hospital stay (14.28±9.403 vs. 14.99±25.037; P=0.719).
Long-term survival
The median survival time for the entire cohort was 59 months (95% CI: 51.159–66.841 months), and the 1-, 3- 5-year survival rates were 85.9%, 63.8%, 47.9%, respectively (Figure 1A). The cumulative survival rates of the patients in the LG did not differ significantly from the survival rates of the patients in the NLG (P=0.076), with 5-year survival rates of 52.4% and 44.7%, respectively (Figure 1B). After PSM, the 1-, 3-, and 5-year survival rates were 87.0%, 64.1%, and 50.9% in the LG, respectively, compared to 85.4%, 59.9%, and 42.3% respectively in the NLG (Figure 1C), and this difference was not statistically significant (P=0.156).

Univariate and multivariate analyses of prognostic factors
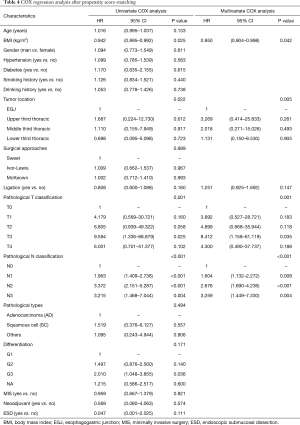
Before matching, univariate Cox regression analyses showed that pathological T stage and pathological N stages were significantly associated with OS. Hence, pT and pN stages, as well as ligation, were included in the multivariate analysis. The list of independent risk factors was narrowed to pT stage (P=0.024) and pN stage (P<0.001). LTD was not a significant prognostic factor in the univariate and multivariate analyses (Table 3). After PSM, BMI, tumor location, pT stage, and pN stage were correlated to OS in the univariate analyses (Table 4). In the multivariate analyses, ligation was still not significantly associated with OS (OR, 1.251; 95% CI: 0.925–1.692, P=0.147).

Full table
Full table
Discussion
In this study, we compared the postoperative complications and long-term survival rates between the thoracic duct ligation group and the non-ligation group. Distinct from the study by Hou et al., this study showed that LTD had no significant impact on postoperative complications or long-term survival in patients with esophageal cancer.
Postoperative chylothorax after esophagectomy is a tremendous burden for patients. Approximately 70% of patients require a second surgical treatment (1), and chylothorax-induced mortality rates can reach as high as 20% (9,10). Since 1948, LTD has been confirmed as an effective approach to treating postoperative chylothorax. To further reduce the occurrence of chylothorax, some surgeons have proposed prophylactic ligation of the thoracic duct (PLTD), where direct or en masse LTD (11) should performed as the routine procedure without considering whether or not the TD is damaged. A recent meta-analysis including 7 clinical studies and 5,254 patients confirmed the utility of PLTD in reducing chylothorax (12). In our study, we performed LTD when intraoperative chylous leakage occurred, or injury to the thoracic duct was highly suspected. Result showed that there was no significant difference in chylothorax incidence between the two groups (1.1% vs. 1.6%; P=1.000). All 3 patients with postoperative chylothorax in the NLG underwent conservative treatment, with a success rate of 100% (3/3), whereas the success rate of conservative treatment in the LG was 50% (1/2). Those may be attributed to the low incidence of postoperative chylothorax (5/609; 0.82%) and small number of enrolled patients. Except for chylothorax, no significant differences between the groups were found for the incidence of complications, which is consistent with a study by Guo et al. (13). Overall, LTD did not increase postoperative complications.
Most of the existing studies emphasize the prevention of postoperative chylothorax, while neglecting other potential consequences of LTD (7). The impact of LTD on long-term survival has yet to be comprehensively understood. Hou et al. compared the survival rates of patients in the LG and NLG, and found that LTD could reduce the OS of patients with esophageal cancer (8). Mechanistically, they attributed the decreased OS in the LG to the loss of immune factors, lipids, and proteins caused by LTD, which warrants further investigation. Growing evidence contradicts these findings. Ehrenhaft et al. (14) reported that blood fat levels dropped significantly after LTD; however, they gradually rose again to baseline levels within 2 weeks. In a study by Liu et al. (15), blood LDL levels gradually recovered within 3 months after decreasing in the first month, while cholesterol, triglycerides, and HDL showed no significant differences between the two groups. In terms of lymphocyte loss, Lee et al. found that lymphocyte counts were significantly reduced after LTD but recovered to normal levels after 3 weeks (16). Taken together, these studies suggest that the metabolic and immunologic changes caused by LTD might be transient. In addition, there was significant heterogeneity between the variables of the LG and NLG in Hou et al.’s study, which might reduce the credibility of the results.
In our study, 1:1 propensity score matching was used to minimize the potential differences between the two groups. We observed that LTD had no obvious influence on OS neither before nor after PSM. Moreover, univariate and multivariate Cox analyses demonstrated that LTD was not a significant prognostic factor, which further validated our primary endpoint findings. Although there were no significant differences between the two groups, Kaplan-Meier plots demonstrated that the 5-year survival rate of the LG was greater than that of the NLG. This could be explained by the fact that the NLG had more patients with pN3 stage and comorbidities, while the LG had more patients with N0–1, G1, and MIS.
In clinical practice, LTD is usually well tolerated due to collateral circulations or the opening of lymphaticovenous communications (17). Although the thoracic duct usually has only one main channel, multiple channels with significant variation can be found in 40% cases (18). In a study by Davis et al. (19), the thoracic ducts of 27.27% patients began in the abdominal cavity as the two channels extended cephalad through the thorax, lying on each side of the aorta, with cross anastomosing channels connecting the two ducts. In addition, there are 3 types of lymphaticovenous communications (LVC) in humans: the central LVC (the termination of thoracic duct), peripheral LVC (communication between lymphatic vessels and veins), and the LVC within lymph nodes (17,20-23). Once the thoracic duct is ligated, the latter two types play a compensatory role in lymphatic return. Peripheral LVCs exist between the lymphatic system and different veins such as the portal vein, the inferior vena cava, the azygos, and the superior vena cava (17). Due to regional obstruction of the lymphatic system, peripheral LVCs were also found in the thigh, leg, and foot (24,25). Located within the paracortex of the lymph nodes are high endothelial venules (HEV), which are specialized vessels that enable direct communication between the lymph and the blood (20). Under pathological conditions, large LVCs within the medulla of lymph nodes may occur once valve incompetency or lymphatic failure takes place (17). Therefore, after LTD, peripheral LVC or lymph node LVC plays a compensatory role in lymphatic circulation.
This study has several limitations. Firstly, the single-center, retrospective nature of this study may have introduced selection bias. Well-designed multi-center randomized controlled trials are needed to validate our results. Secondly, PSM excluded unmatched individuals from the analysis, which reduced the sample size and affected the results. Thirdly, due to difficulty during follow-up, we did not analyze indicators that are reflective of nutritional status.
In conclusion, our study demonstrated that LTD does not increase postoperative complications, and there is no significant correlation between LTD and the prognosis of patients who undergo esophagectomy.
Acknowledgments
Funding: This work was supported by the National Natural Science Foundation of China (grant number 81472688).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/jtd-20-1341
Data Sharing Statement: Available at http://dx.doi.org/10.21037/jtd-20-1341
Peer Review File: Available at Available at http://dx.doi.org/10.21037/jtd-20-1341
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd-20-1341). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Ethics Committee of Changhai Hospital (CHEC-2019-359) and informed consent was taken from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Chen KN. Managing complications I: leaks, strictures, emptying, reflux, chylothorax. J Thorac Dis 2014.S355-63. [PubMed]
- Miao L, Zhang Y, Hu H, et al. Incidence and management of chylothorax after esophagectomy. Thorac Cancer 2015;6:354-8. [Crossref] [PubMed]
- Bryant AS, Minnich DJ, Wei B, et al. The incidence and management of postoperative chylothorax after pulmonary resection and thoracic mediastinal lymph node dissection. Ann Thorac Surg 2014;98:232-5; discussion 235-7. [Crossref] [PubMed]
- Kranzfelder M, Gertler R, Hapfelmeier A, et al. Chylothorax after esophagectomy for cancer: impact of the surgical approach and neoadjuvant treatment: systematic review and institutional analysis. Surg Endosc 2013;27:3530-8. [Crossref] [PubMed]
- Sauvanet A. Surgery technique: preventive ligation of the thoracic duct during esophagectomy for cancer. Ann Chir 2002;127:228-31. [Crossref] [PubMed]
- Lampson RS. Traumatic chylothorax; a review of the literature and report of a case treated by mediastinal ligation of the thoracic duct. J Thorac Surg 1948;17:778-91. [Crossref] [PubMed]
- Choh CT, Khan OA, Rychlik IJ, et al. Does ligation of the thoracic duct during oesophagectomy reduce the incidence of post-operative chylothorax? Int J Surg 2012;10:203-5. [Crossref] [PubMed]
- Hou X, Fu JH, Wang X, et al. Prophylactic thoracic duct ligation has unfavorable impact on overall survival in patients with resectable oesophageal cancer. Eur J Surg Oncol 2014;40:1756-62. [Crossref] [PubMed]
- Mishra PK, Saluja SS, Ramaswamy D, et al. Thoracic duct injury following esophagectomy in carcinoma of the esophagus: ligation by the abdominal approach. World J Surg 2013;37:141-6. [Crossref] [PubMed]
- Shah RD, Luketich JD, Schuchert MJ, et al. Postesophagectomy chylothorax: incidence, risk factors, and outcomes. Ann Thorac Surg 2012;93:897-903; discussion 903-4. [Crossref] [PubMed]
- Lin Y, Li Z, Li G, et al. Selective En Masse Ligation of the Thoracic Duct to Prevent Chyle Leak After Esophagectomy. Ann Thorac Surg 2017;103:1802-7. [Crossref] [PubMed]
- Crucitti P, Mangiameli G, Petitti T, et al. Does prophylactic ligation of the thoracic duct reduce chylothorax rates in patients undergoing oesophagectomy? A systematic review and meta-analysis. Eur J Cardiothorac Surg 2016;50:1019-24. [Crossref] [PubMed]
- Guo W, Zhao YP, Jiang YG, et al. Prevention of postoperative chylothorax with thoracic duct ligation during video-assisted thoracoscopic esophagectomy for cancer. Surg Endosc 2012;26:1332-6. [Crossref] [PubMed]
- Ehrenhaft JL, Meyers R. Blood fat levels following supradiaphragmatic ligation of the thoracic duct. Ann Surg 1948;128:38-45. [Crossref]
- Liu JP, Zhang YH, Yang B, et al. Influence of thoracic duct ligation on the lipid metabolism of patients with esophageal carcinoma after esophagectomy. Genet Mol Res 2015;14:2527-36. [Crossref] [PubMed]
- Lee FC. Changes in the number of small lymphocyte of the blood following ligation of the thoracic duct. J Exp Med 1922;36:247-60. [Crossref] [PubMed]
- Hidden G, Menard P, Zorn JY. Lymphaticovenous communications. Role of the lymph nodes. Anat Clin 1985;7:83-91. [Crossref] [PubMed]
- Phang K, Bowman M, Phillips A, et al. Review of thoracic duct anatomical variations and clinical implications. Clin Anat 2014;27:637-44. [Crossref] [PubMed]
- Davis HK. A Statistical Study of the Thoracic Duct in Man. Am J Anat 1915;17:211-44. [Crossref]
- Miranda Garcés M, Mirapeix R, Pons G, et al. A comprehensive review of the natural lymphaticovenous communications and their role in lymphedema surgery. J Surg Oncol 2016;113:374-80. [Crossref] [PubMed]
- Heymans O, Fallais C, Hustinx R. Intratissular lymphaticovenous anastomoses demonstrated by perioperative intramuscular injection of 99mTC-colloids. Lymphat Res Biol 2006;4:29-33. [Crossref] [PubMed]
- Stamp GF, Peters AM. Peripheral lymphovenous communication in lymphoedema. Nucl Med Commun 2012;33:701-7. [Crossref] [PubMed]
- Koehler PR, Schaffer B. Peripheral lymphatico-venous anastomoses. Report of two cases. Circulation 1967;35:401-4. [Crossref] [PubMed]
- Kariya S, Komemushi A, Nakatani M. Intranodal lymphangiogram: technical aspects and findings. Cardiovasc Intervent Radiol 2014;37:1606-10. [Crossref] [PubMed]
- Threefoot SA, Kossover MF, Kent WT, et al. Factors stimulating function of lymphaticovenous communications. Angiology 1967;18:682-98. [Crossref] [PubMed]


