
Minimally invasive ventricular assist device implantation
Introduction
The use of mechanical circulatory support (MCS) in terminal heart failure patients has been established as an important therapeutic option to improve quality of life and to prevent potentially fatal end-organ dysfunction (1). The need for MCS has increased in recent years due to a general shortage of donor organs particularly in Europe (2). Further, MCS therapy is considered as a vital option for patients not meeting the usual heart transplantation criteria, so called destination therapy concept. Ventricular assist devices (VADs) have traditionally been implanted through median sternotomy (MS). However, the introduction of the 3rd generation centrifugal pumps and accompanying improvement in pump design led to evolvement of new surgical techniques aiming to reduce the invasiveness of the procedure and lower the cardiopulmonary bypass (CPB) related complications (3,4).
A series of review studies (5,6) and the recently published multi-center LATERAL trial (7) have shown that a less invasive approach for implanting left ventricular assist devices (LVADs), with or without using of CPB machine, results in fewer blood transfusions, less hemodialysis, significantly shorter intensive-care unit and hospital stays, as well as lower rates of right ventricular (RV) failure, while having no higher rates of adverse events compared to the standard approach (8-10). In this article we aim to provide an overview of the currently available less-invasive surgical techniques while discussing their benefits and possible drawbacks, with particular focus on intra-pericardial centrifugal pumps.
History of less-invasive approach for VAD implantation
While the traditional approach for VAD implantation in many centers worldwide is the sternotomy approach, wide opening of the pericardium, application of CPB and cardiac luxation for pump implantation, the less invasive approach aims to reduce some of the above mentioned surgical trauma and aims to keep the pericardium closed. The definition of less-invasive implant strategies for LVADs has been vague. Usually it involves minimizing or completely avoiding sternal trauma, avoiding heart luxation while simultaneously leaving the major part of pericardium intact. The first less-invasive cases were published by Pasic et al. in 1999 with the successful implantation of a paracorporeal LVAD through left antero-lateral thoracotomy in patients with high perioperative risk due to multiple previous cardiac operations (11). Further, Hill et al. described further cases of minimal invasive approach to LVAD implantation in year 2000 combining right mini-thoracotomy with left subcostal incision for a paracorporeal Thoratec LVAD (Thoratec Corporation, Pleasanton, USA) (12).
The idea of a less invasive implanting procedure of intracorporeal LVADs for patients with severe comorbidities has been discussed and applied in practice as early as 2003 by Frazier et al., where an axial-flow Jarvik-2000 LVAD was implanted in a patient through left-lateral thoracotomy using an anastomosis into the descending aorta without the need for CPB (13). Additional reports from 2008 by Gregoric et al. on minimally-invasive implantation of axial-flow Thoratec Heart Mate II (HM2) LVADs in six patients through right mini-thoracotomy and left sub-costal incision showed a safe and reliable outcome (14).
Needless to say, the application of less invasive approaches for VAD surgery remained sporadic and limited to anecdotal case report until the introduction of the intra-pericardial centrifugal pumps starting with HeartWare HVAD (HeartWare Inc, Framingham, MA) and followed by Heart Mate III (Abbott Laboratories, Lake Bluff, IL). The smaller size of these pumps, and the attachment of the inflow cannula to the pump facilitates implantation using less invasive approaches. The first report on off-pump HVAD implantation using hemi-sternotomy and antero-lateral thoracotomy was published 2011 by Cheung et al. in Australia (15). Afterwards, many centers started using these less invasive techniques for VAD implantation without notable evidence or robust data supporting this approach. Some clinicians started even using these less invasive approaches successively for redo cases and/or for pump explant and/or exchange with a reliable outcome (16). In a recent case report even anticoagulant-free off-pump LVAD implant was described (17).
Surgical technique
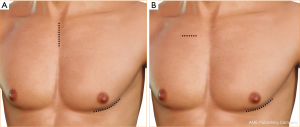
There are two main techniques described for less invasive centrifugal VAD implantation. The first and the most commonly used technique is hemi-sternotomy and left antero-lateral thoracotomy approach. The second technique is the so called sternum sparing technique with one right mini-thoracotomy incision (traditionally 2nd intercostal space) and another left antero-lateral thoracotomy incision (Figure 1). In both techniques the patient is positioned supine and a possible 30-degree rotation to the right can be used to allow for better exposure of the thoracotomy. The sterile field is always prepared as for a full sternotomy. A J-shaped upper hemi-sternotomy is performed up to the 2nd or 3rd intercostal space, according to the position of the aorta. Another alternative technique is the so called “inverted Y” technique, however, some reports indicate that this approach may decrease chest wall stability after minimally-invasive aortic valve replacement surgery (18). For sternum sparing technique, an incision is performed at the right 2nd intercostal space to achieve outflow graft (OG) anastamosis.
The left antero-lateral thoracotomy is similar in both approaches. The exact localization of the antero-lateral thoracotomy is facilitated using trans-thoracic echocardiography (TTE) guidance. The correct spot for the LV core can be identified by poking a finger into the LV apex and checking the corresponding position through a trans-esophageal echocardiogram (TEE). A 3D image of the TEE may be helpful. The inflow sewing ring is then sutured to the heart ideally without using the CPB machine (Figure 2A). At this point, the patient can be fully heparinized and CPB can be started using venous cannulation into the right femoral vein and arterial cannulation into the ascending aorta. For sternum sparing approach cannulation of the femoral vessels is necessary. The LV apex is then cored for pump placement, the LV inspected for thrombus or trabeculae, and the pump is placed and secured. If no CPB machine is used, the LV coring and pump insertion is the most critical part of the procedure. Beating heart, use of adenosine or rapid pacing have been described for use during this maneuver (8).
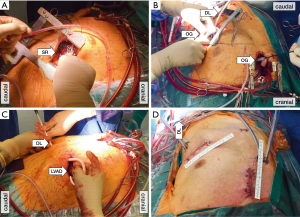
The OG is tunneled intra-pericardially lateral to the right ventricle towards the ascending aorta, if there hasn’t been a previous sternotomy. This is performed through a pericardial incision close to the aortic base. A surgical forceps is inserted into the incision and the free end of the OG is bound with thick silk suture, which is then grasped and pulled from the apical pericardial incision towards the aorta (Figure 2B). In case of a previous sternotomy, the OG may be tunneled extra-pericardially medial to the left lung to avoid laceration of cardiac structures (particularly in the presence of open LIMA-LAD bypass) due to expected adhesions. Alternative approaches for OG anastomose include: connecting the OG to the ascending aorta using the aforementioned sternum sparing right antero-lateral thoracotomy (19), anastomosing the OG to the ventral aortic arch (20) or the descending aorta, both without the need of a second thoracic incision (14) or attaching the OG to the left subclavian artery using a second left subclavian incision (8,21). The latter is of particular advantage in patients with heavily calcified ascending aorta.
The driveline is then tunneled ideally using a dual-incision modified long subfascial C-shaped technique, also called “double tunnel technique”, as described by Schibilsky et al. (Figure 2C). Compared to the manufacturers’ recommended short straight tunneling procedure to the right subcostal space, this approach has shown to decrease the rate of infection while providing more surgical option in case of driveline exit-site infections (22). Regardless of the technique being used, it is important to consider closing or adapting of the pericardial layer at the end of the procedure. This will facilitate future procedures such as heart transplantation and has the theoretical advantage of boosting right ventricular function (for post-operative result, see Figure 2D).
Advances in robot-assisted surgeries have been reported as well, performing implantation through a short left antero-lateral thoracotomy and a right anterior thoracotomy has been described with excellent results (23), however at present, these cases are very limited and the DaVinci robot system (Intuitive Surgical Inc., USA) is not widely in use for cardiac surgery.
The necessity of CPB
The less invasive VAD implantation may be performed with or without using the CPB machine. Placing the patient on CPB during the minimally invasive LVAD implantation offers several advantages. For example, manipulation of the heart under CPB avoids hemodynamic instability, and possible complications are easier to manage. The main advantage is the possibility to inspect the left ventricle at the time of LV coring. This may be helpful in avoiding stroke and pump thrombosis. Further, de-airing of the pump may be easier when CPB is used. Notably, no data exits supporting the myth that off-pump VAD implantation is associated with higher rates of stroke and/or pump thrombosis.
Meanwhile, an alternative off-pump VAD implantation has been postulated to avoid possible CPB drawbacks (24). Some authors support the idea that off-pump LVAD implantation performed in experienced centers might reduce hemodilution, systemic inflammatory response syndrome, negative effects of pulmonary hypertension and postoperative RV dysfunction (8,25,26). While off-pump less invasive VAD implantation can be a useful tool, special care should be taken during the apical coring procedure. To mitigate the blood loss, adenosine can be administered, or a short phase of induced ventricular tachycardia may be helpful. Our experience showed that performing the coring on beating heart may be more advantageous and hemodynamically better tolerated than rapid pacing or adenosine administration (26).
There are only limited studies on off-pump VAD implantation. In a study by Sileshi et al. on-pump conventional sternotomy had a significantly longer duration of inotropes compared with the off-pump group (25). In another study by Gregoric et al. the off-pump approach allowed HeartWare HVAD to be implanted faster, with significantly less perioperative bleeding and transfusion requirements, and facilitates postoperative rehabilitation (27). Due to the lack of direct visualization of the left ventricle in off-pump VAD implantation cases, extreme care must be given to rule out any thrombus within the left ventricle using TEE. Further, in our experience, the current coring tools allow easier off-pump implantation when HeartWare HVAD is used. Off-pump implantation of HeartMate 3 (HM3) using the current tools is very challenging. Alternatively, the coring knife of HeartWare HVAD may be used (off-label) for HM3 implantation. This approach was used from our group for the first off-pump implantation of HM3 worldwide (26).
Concomitant procedures at the time of less invasive VAD implantation
There may be some necessary concomitant procedures that need to be performed at the time of VAD implantation. These usually include patent foramen ovale closure, aortic valve repair/replacement, coronary bypass surgery, tricuspid valve repair and/or mitral valve repair. The majority of surgeons prefers full sternotomy if concomitant procedures are required. As a matter of fact, aortic valve surgery may be performed using the same hemi-sternotomy approach used for VAD implantation (19). However, patent foramen ovale closure and mitral and tricuspid valve surgery almost always require full sternotomy. The current guidelines suggest foramen ovale closure at the time of VAD implantation surgery. However, data regarding tricuspid and mitral valve surgery is scarce and controversial (28).
Never the less, Hillebrand et al. reported 4 cases of less invasive LVAD-Implantation and concomitant TVR using Cosgrove-Edwards partial flexible annuloplasty ring for tricuspid valve regurgitation through upper hemi-sternotomy under complete CPB. The survival rate remained 100% for these patients (29). Further, Schaefer et al. reported a case of successful less invasive HeartWare HVAD Implantation and simultaneous trans-apical transcatheter aortic valve replacement using a JenaValve (JenaValve Technologies Inc., Munich, DE) in a patient with ischemic cardiomyopathy and aortic valve regurgitation through right anterior and left anterior thoracotomies. The LVAD sewing ring was placed under CPB support. An insertion of the valve delivery system took place through the LVAD sewing ring resulting in significantly shorter operation time. There were no major or minor complications in the follow-up period (30). Meanwhile, Orasanu et al. reported a case of LVAD implantation with concomitant valve-in-valve transcatheter mitral valve replacement via transapical approach on CPB in a patient with mitral prosthesis degeneration and ischemic cardiomyopathy. This patient, too, remained ambulatory and full asymptomatic in the follow-up period (31). In summary, except for aortic valve procedures, the majority of surgeons tends to use full sternotomy approach when concomitant procedures are necessary at the time of VAD implantation.
Less-invasive LVAD for redo cases and VAD exchange or explantation
We believe that less invasive VAD implantation is particularly useful for redo cases, especially in the presence of open arterial or venous grafts. The main advantage here is avoiding the preparation and injury of the right ventricle and/or grafts. Therefore bleeding and transfusion requirements may be significantly lowered. Notably, there is no published data supporting this finding but some groups, including ours, are currently aiming to prove this finding. As a matter of fact, there is usually minimal adhesions at the LV apex which makes the anterolateral incision straight forward. However, the caution is mandatory at the time of hemisternotomy. In the presence of open arterial grafts to left anterior descending artery, we traditionally tunnel the OG outside of pericardium and medial to the left lung side to allow the OG anastomose to the ascending aorta.
Less invasive surgery (LIS) may be also considered for pump exchange in selected cases. The advantage is avoiding right ventricular adhesions which is necessary for sternotomy cases. Preoperative patient selection is very important. These procedures have to be well planned including a CT scan performed prior to the procedure. Access to the pump needs to be carefully evaluated. Few cases of HeartWare HVAD and HeartMate 3 pump exchange for pump thrombosis via redo left thoracotomy through the 5th intercostal space were reported with good results (16,32). Meanwhile, Louis et al. reported a case of LVAD exchange of a HM3 for pump thrombosis via lateral bilateral minithoracotomies. The LVAD had initially been implanted via full sternotomy. An incision was made in the left 5th intercostal space and the right 2nd intercostal space. After encountering significant adhesions, the HM3 pump was exposed and removed from the apex of the heart. For the OG, a graft-to-graft anastomosis was performed to minimize manipulation of the aorta and avoid any trauma that might be caused by clamping of the aorta. The patient remained asymptomatic at the 2-month follow-up (33).
The right ventricle and less invasive VAD implantation approach
RV failure has been described as one of the most unpredictable and frequent complications after LVAD implantation. Severe RV failure requiring the implant of a right ventricle assist device (RVAD) is associated with a reduced short-term and long-term survival which is only 58% at 1 year and 31% at 5 years (34). It has been postulated that less invasive VAD implantation may reduce the incidence and/or severity of right ventricular failure (10). The theoretical advantages include the preservation of the RV geometry during surgery by avoiding cardiac luxation and preserving the pericardium. The possible protective role of less invasive VAD surgery has been shown in several studies by the low incidence of postoperative RV failure and reduced need for RV assist devices (7,35-39).
Gosev et al. compared the results between sternal-sparing and conventional techniques in 105 patients implanted with HM3 LVAD. CPB was utilized for all patients in this study (40). In the less-invasive approach, small bilateral thoracotomies were utilized in the left 5th or 6th and right 2nd intercostal spaces with the pericardium being left intact around the ventricle. Despite similar baseline characteristics between the cohorts, the sternal-sparing group demonstrated significantly fewer blood-product transfusions, a shorter length of hospital stay and lower incidence of severe right ventricular failure.
Furthermore, our group has shown that in a matched group of patients (less invasive vs. sternotomy VAD implantation), the bleeding and rethoracotomy rate is lower in less invasive VAD implantation cases. Further, the length of hospital stay is shorter and better postoperative RV function was observed (10).
Needless to say, even with less invasive VAD implantation, RV failure may still occur. There are many options how to support the RV in these patients. These include:
Attaching the OG to pulmonary artery through the hemi-sternotomy approach and percutaneous cannulation of the femoral vein (venous cannule) (41).
Using ProtekDuo TandemHeart™ Pump (LivaNova PLC, London, UK) for RV support.
Using Impella® RP (Abiomed, Inc., Danvers, MA, USA) which is positioned percutaneously through the femoral vein.
The least attractive option is to implant veno-arterial extra corporeal membrane oxygenation as a form of RV support.
Summary and conclusions
The emergence of the new generation intra-pericardial centrifugal pumps was accompanied by introduction of alternative less invasive surgical approaches that reduce surgical trauma and may minimize postoperative morbidities. The majority of VAD implantations worldwide are still performed using full sternotomy approach. Many centers have started using the less invasive approach. This led to initiation of LATERAL Trial, which contributed to FDA and CE approval of less invasive approach for HeartWare HVAD implantation (7). Further, supportive data from Elevate Register facilitated FDA and CE approval of less invasive HM3 implantation as well (42,43). Less invasive VAD implantation may be performed with or without CPB machine. However, the current surgical tools allow easier off-pump insertion of HeartWare HVAD compared to HM3. The majority of the centers use the hemi-sternotomy approach. However, some centers use right thoracotomy instead of hemi-sternotomy approach. When concomitant procedures are necessary, full sternotomy may be the better option. The available literature supports that less invasive VAD implantation causes less RV failure, bleeding and rethoracotomy as well as shorter hospital stays compared to sternotomy approach. In our experience, the advantages of less invasive VAD implantation is particularly observed in redo cases and at the time of future surgeries for instance pump exchange and /or heart transplantation. Therefore, less invasive VAD implantation considered as standard VAD implantation approach at our center.
Acknowledgments
The authors would like to thank their spouses and colleagues for their ongoing support.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Jason Ali and Yasir Abu-Omar) for the series “Minimally Invasive Cardiac Surgery” published in Journal of Thoracic Disease. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd-20-1492). The series “Minimally Invasive Cardiac Surgery” was commissioned by the editorial office without any funding or sponsorship. Michael Borger serves as an unpaid editorial board member of Journal of Thoracic Disease from Sep 2018 to Aug 2020 and reports that his hospital receives modest (< $10,000 per annum) speakers‘ honoraria and/or consulting fees on his behalf from Edwards Lifesciences, Medtronic, Abbott, CryoLife, and Ascyrus. However, these are all related to heart valve therapy and not to LVAD therapy. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Frazier OH, Rose EA, McCarthy P, et al. Improved Mortality and Rehabilitation of Transplant Candidates Treated with a Long-Term Implantable Left Ventricular Assist System Ann Surg 1995;222:327-36. [Crossref] [PubMed]
- Branger P, Samuel U. Annual Report. Eurotransplant Foundation, 2017.
- Collart F. A safe, alternative technique for off-pump left ventricular assist device implantation in high-risk reoperative cases. Interact Cardiovasc Thorac Surg 2004;3:286-8. [Crossref] [PubMed]
- Weiland AP, Walker WE. Physiologic principles and clinical sequelae of cardiopulmonary bypass. Heart Lung 1986;15:34-9. [PubMed]
- Wachter K, Franke UFW, Rustenbach CJ, et al. Minimally Invasive versus Conventional LVAD-Implantation-An Analysis of the Literature. Thorac Cardiovasc Surg 2019;67:156-63. [Crossref] [PubMed]
- Ricklefs M, Hanke JS, Dogan G, et al. Less Invasive Surgical Approaches for Left Ventricular Assist Device Implantation. Semin Thorac Cardiovasc Surg 2018;30:1-6. [Crossref] [PubMed]
- McGee E, Danter M, Strueber M, et al. Evaluation of a lateral thoracotomy implant approach for a centrifugal-flow left ventricular assist device: The LATERAL clinical trial. J Heart Lung Transplant 2019;38:344-51. [Crossref] [PubMed]
- Strueber M, Meyer AL, Feussner M, et al. A minimally invasive off-pump implantation technique for continuous-flow left ventricular assist devices: early experience. J Heart Lung Transplant 2014;33:851-6. [Crossref] [PubMed]
- Ayers B, Sagebin F, Wood K, et al. Complete Sternal-Sparing Approach Improves Outcomes for Left Ventricular Assist Device Implantation in Patients With History of Prior Sternotomy. Innovations (Phila) 2020;15:51-6. [Crossref] [PubMed]
- Saeed D, Jawad K, Sipahi NF, et al. Reduced rethoracotomy rate for bleeding and postoperative hospital stay with Less invasive ventricular assist device implantation: Multicenter Experience. J Heart Lung Transplant 2020;39:S149-50. [Crossref]
- Pasic M, Bergs P, Hennig E, et al. Simplified technique for implantation of a left ventricular assist system after previous cardiac operations. Ann Thorac Surg 1999;67:562-4. [Crossref] [PubMed]
- Hill JD, Avery GJ, Egrie G, et al. Less invasive Thoratec LVAD insertion: a surgical technique. Heart Surg Forum 2000;3:218-23. [PubMed]
- Frazier OH. Implantation of the Jarvik 2000 left ventricular assist device without the use of cardiopulmonary bypass. Ann Thorac Surg 2003;75:1028-30. [Crossref] [PubMed]
- Gregoric ID, La Francesca S, Myers T, et al. A less invasive approach to axial flow pump insertion. J Heart Lung Transplant 2008;27:423-6. [Crossref] [PubMed]
- Cheung A, Lamarche Y, Kaan A, et al. Off-pump implantation of the HeartWare HVAD left ventricular assist device through minimally invasive incisions. Ann Thorac Surg 2011;91:1294-6. [Crossref] [PubMed]
- Sajjad M, Butt T, Oezalp F, et al. An alternative approach to explantation and exchange of the HeartWare left ventricular assist device. Eur J Cardiothorac Surg 2013;43:1247-50. [Crossref] [PubMed]
- Ali J, Balasubramanian S, Berman M, et al. Anticoagulant-Free Off-Pump Left Ventricular Assist Device Implant. Ann Thorac Surg 2018;105:e37-9. [Crossref] [PubMed]
- Pfeiffer S, Fischlein T, Vogt F, et al. Superior vena cava cannulation in aortic valve surgery: an alternative strategy for a hemisternotomy approach. Interact Cardiovasc Thorac Surg 2015;20:863-5. [Crossref] [PubMed]
- Haberl T, Riebandt J, Mahr S, et al. Viennese approach to minimize the invasiveness of ventricular assist device implantation†. Eur J Cardiothorac Surg 2014;46:991-6; discussion 996. [Crossref] [PubMed]
- Popov AF, Mohite PN, Sabashnikov A, et al. Minimally invasive HeartWare LVAD implantation through single left thoracotomy. J Artif Organs 2015;18:170-2. [Crossref] [PubMed]
- Carrozzini M, Bejko J, Guariento A, et al. Minimally Invasive Implantation of Continuous Flow Left Ventricular Assist Devices: The Evolution of Surgical Techniques in a Single-Center Experience. Artif Organs 2019;43:E41-52. [Crossref] [PubMed]
- Schibilsky D, Benk C, Haller C, et al. Double tunnel technique for the LVAD driveline: improved management regarding driveline infections. J Artif Organs 2012;15:44-8. [Crossref] [PubMed]
- Khalpey Z, Sydow N, Slepian MJ, et al. How to do it: thoracoscopic left ventricular assist device implantation using robot assistance. J Thorac Cardiovasc Surg 2014;147:1423-5. [Crossref] [PubMed]
- Abdeen MSKM, Albert A, Maxhera B, et al. Implanting permanent left ventricular assist devices in patients on venoarterial extracorporeal membrane oxygenation support: do we really need a cardiopulmonary bypass machine? Eur J Cardiothorac Surg 2016;50:542-7. [Crossref] [PubMed]
- Sileshi B, Haglund NA, Davis ME, et al. In-hospital outcomes of a minimally invasive off-pump left thoracotomy approach using a centrifugal continuous-flow left ventricular assist device. J Heart Lung Transplant 2015;34:107-12. [Crossref] [PubMed]
- Saeed D, Sixt S, Albert A, et al. Minimally invasive off-pump implantation of HeartMate 3 left ventricular assist device. J Thorac Cardiovasc Surg 2016;152:1446-7. [Crossref] [PubMed]
- Gregoric ID, Radovancevic R, Akay MH, et al. Short-Term Experience with Off-Pump Versus On-Pump Implantation of the HeartWare Left Ventricular Assist Device. ASAIO J 2017;63:68-72. [Crossref] [PubMed]
- Saeed D, Kidambi T, Shalli S, et al. Tricuspid valve repair with left ventricular assist device implantation: is it warranted? J. Heart Lung Transplant 2011;30:530-5. [Crossref] [PubMed]
- Hillebrand J, Hoffmeier A, Djie Tiong Tjan T, et al. Minimally Invasive Implantation of HeartWare Assist Device and Simultaneous Tricuspid Valve Reconstruction Through Partial Upper Sternotomy. Artif Organs 2017;41:418-23. [Crossref] [PubMed]
- Schaefer A, Treede H, Bernhardt A, et al. Concomitant minimally invasive HVAD and transapical aortic valve implantation. ASAIO J 2015;61:209-12. [Crossref] [PubMed]
- Orasanu G, Al-Kindi SG, Robinson MR, et al. First-in-Human Experience With Transcatheter Mitral Valve-in-Valve Implantation During Left Ventricular Assist Device Placement. Circ Heart Fail 2016;9:e003458 [Crossref] [PubMed]
- Radwan M, Risteski P, Hoffmann R, et al. Repeat left ventricular assist device exchange with inflow or outflow correction for recurrent pump thrombosis and cerebral haemorrhage through limited incisions. Eur J Cardiothorac Surg 2018;54:781-3. [Crossref] [PubMed]
- Louis C, Ayers B, Barrus B, et al. HeartMate 3 pump exchange via sternal-sparing bilateral minithoracotomies. J Card Surg 2020;35:901-3. [Crossref] [PubMed]
- Kormos RL, Cowger J, Pagani FD, et al. The Society of Thoracic Surgeons Intermacs database annual report: Evolving indications, outcomes, and scientific partnerships. J Heart Lung Transplant 2019;38:114-26. [Crossref] [PubMed]
- Kocabeyoglu SS, Kervan U, Sert DE, et al. Is it Possible to Implant HeartMate 3 Less Invasively? New Pump, New Approach. Artif Organs 2018;42:1132-8. [Crossref] [PubMed]
- Wood KL, Ayers BC, Sagebin F, et al. Complete Sternal-Sparing HeartMate 3 Implantation: A Case Series of 10 Consecutive Patients. Ann Thorac Surg 2019;107:1160-5. [Crossref] [PubMed]
- Mohite PN, Sabashnikov A, Raj B, et al. Minimally Invasive Left Ventricular Assist Device Implantation: A Comparative Study. Artif Organs 2018;42:1125-31. [Crossref] [PubMed]
- Pasrija C, Sawan MA, Sorensen E, et al. Less invasive left ventricular assist device implantation may reduce right ventricular failure. Interact Cardiovasc Thorac Surg 2019;29:592-8. [Crossref] [PubMed]
- Maltais S, Anwer LA, Tchantchaleishvili V, et al. Left Lateral Thoracotomy for Centrifugal Continuous-Flow Left Ventricular Assist Device Placement: An Analysis from the Mechanical Circulatory Support Research Network. ASAIO J 2018;64:715-20. [Crossref] [PubMed]
- Gosev I, Wood K, Ayers B, et al. Implantation of a fully magnetically levitated left ventricular assist device using a sternal-sparing surgical technique. J Heart Lung Transplant 2020;39:37-44. [Crossref] [PubMed]
- Maxhera B, Albert A, Westenfeld R, et al. Minimally Invasive Right Ventricular Assist Device Implantation in a Patient with HeartWare left ventricular Assist Device. ASAIO J 2015;61:e42-3. [Crossref] [PubMed]
- Gustafsson F, Shaw S, Lavee J, et al. Six-month outcomes after treatment of advanced heart failure with a full magnetically levitated continuous flow left ventricular assist device: report from the ELEVATE registry. Eur Heart J 2018;39:3454-60. [Crossref] [PubMed]
- Garbade J, Gustafsson F, Shaw S, et al. Postmarket Experience With HeartMate 3 Left Ventricular Assist Device: 30-Day Outcomes From the ELE-VATE Registry. Ann Thorac Surg 2019;107:33-9. [Crossref] [PubMed]


