|
Review Article
Cryosurgery for lung cancer
Lizhi Niu1,2, Kecheng Xu1,2, Feng Mu1,2
1Department of Oncology, Affiliated Fuda Hospital, Guangzhou Institutes of Biomedicine and Health, Chinese Academy of Science, No. 91-93 Judezhong Road, Haizhu District, Guangzhou 510305, China; 2Guangzhou Fuda Cancer Hospital, Jinan University School of Medicine, No. 2 Tangdexi Road, Tianhe District, Guangzhou 510305, China
Corresponding to: Lizhi Niu, MD, PhD. Guangzhou Fuda Cancer Hospital, Jinan University School of Medicine. No. 2 Tangdexi Road, Tianhe District, Guangzhou 510305, China. Email: niuboshi1966@yahoo.com.cn.
|
|
Abstract
Cryosurgery is suited for patients with lung cancer who are not considered for lung resection because of the advanced stage of the disease or the patient’s poor general condition or poor respiratory function and with tumor recurrence following radiotherapy, chemotherapy or lung resection, and those patients who have localized lung cancer but refuse to receive operative therapy. Procedures of cryosurgery for lung cancer can be performed through endobronchial, direct intrathoracic (at exploratory thoracotomy) or percutaneous routes depending upon location and size of tumor. Six hundred and twenty-five patients with Non-small cell lung cancer (NSCLC) received percutaneous cryoablation in Fuda Cancer Hospital Guangzhou, China. One hundred and fifty patients were followed-up for 12 to 38 months. Results showed that 1-, 2-, and 3-year survival rates were 64%, 45% and 32%, respectively. The adverse effects after cryosurgery of lung cancer include haemoptysis, pneumothorax, bloody thorax, pleural effusion and pulmonary infection which are generally mild, transient, and recovery with symptomatic management. In vitro studies have shown cryotherapy of lung cancer cells can improve the immune system to trigger the specific anti-tumor response. In the future, comparative studies between this modality and other therapies should be conducted for the treatment of lung cancer. In addition, more attention needs to be put on the immunomodulators that enhance the cryoimmunology.
Key words
Lung cancer; cryosurgery; cryoablation; cryotherapy; cryoimmunology
J Thorac Dis 2012;4(4):408-419. DOI: 10.3978/j.issn.2072-1439.2012.07.13 |
|
Introduction
Lung cancer is the most common cause of cancer death, with a very poor survival rate. By the time of diagnosis, most cases are at an advanced stage. In recent years, little progress has been made in improving the quality of life of patients with advanced lung cancer ( 1, 2). This has added to the importance of alleviating symptoms and improving quality of life for patients of advanced stage, inoperable carcinoma. Where the possibility of surgery has been eliminated, other palliative measures must be considered. These treatments include radiochem therapy, laser therapy, photodynamic therapy, brachytherapy, radiofrequency ablation (RFA), and cryosurgery ( 3-6). Cryosurgery is a treatment in which tumors are frozen and then left in situ to be reabsorbed. Several publications reported the results of cryosurgery for treatment of carcinoma of prostate, liver, breast and kidneys. Current long-term follow-up study showed that cryosurgery is an important option for a wide range of unresectable cancers and provides the potential for long-term survival ( 7-9). For the past several years, endobronchial cryoablation has been used to treat the patients with inoperable obstructive central bronchial lung tumors and is shown to be effective in reopening obstructed airways ( 9-11). Direct cryoablation has recently been applied for unresectable lung tumors, showing encouraging results ( 10, 12-14). As advances are made in imaging guidance and the improvement of cryosurgical apparatus, percutaneous mode of cryosurgery, a less invasive procedure, has been successfully used for treatment of lung cancer, including early and advanced stage of lesion ( 15). |
|
Indication
Endobronchial cryosurgery is adaptable for treatment for ( 10, 11, 16, 17):
• Histologically proven carcinoma of the trachea and bronchi;
• Inoperable carcinoma based on the position of the tumor,
performance status or poor respiratory function predominantly;
• Intraluminal tumors;
• Extraluminal elements of tumors which do not cause
occlusion from external pressure of more than 75% of the normal
diameter;
• Recurred tumor following radiotherapy, chemotherapy or
lung resection. Direct intrathoracic cryosurgery is adaptable for the patients
whose cancer initially considered to be operable, but were found
to have unresectable tumors at thoracotomy ( 10-14). Percutaneous cryosurgery is adaptable for ( 15-17):
• Small and solitary lung cancer, which cannot receive
operational therapy because of patient’s poor performance and
respiratory function or refuse operation;
• Advanced cancer, which is considered unresectable in term
of tumor size and location;
• Selected cases of centrally-located lung cancer. For the small and solitary lung cancer, the cryosurgery’s
aim can be radical; while for advanced lung cancer, the goal is
debulking of tumor to improve symptoms, quality of life and
survival of patient.
|
|
Technology
Endobronchial cryosurgery
The procedure is performed under short-acting intravenous
general anaesthesia, using a large rigid (9.2 mm) or a flexible
bronchoscope (2.4 mm) ( 10, 11, 16, 17). The distal tip of the
bronchoscope is placed about 5 mm above the lesion and the
appropriate cryoprobe is inserted through the biopsy channel
(bronchoscope) into the tumor. A Joule-Thomson type probe
with argon or nitrous oxide as the cryogen is often used. A
temperature of around –160˚C is achieved at the probe tip.
Careful monitoring of temperature during cryosurgery is carried
out. The tumor is frozen for 3 to 5 minutes and then allowed to
thaw until the probe is separated from the tissue. Two cycles of
freeze/thaw are often performed. Tissue samples for histological
examination are taken before each cryosurgery. For the tumor
covered wider areas of the bronchial tree, multiple cryoapplications
are necessary during the same treatment session.
Necrotic tumor material, when present, is removed after each
cryo-application using a biopsy-type clamp. To treat or prevent
bleeding from the site of a biopsy or cryosurgery, the epinephrine
(adrenaline) 1:1,000 is locally applied. The selection of probe diameter (2.2 or 5 mm) and shape
(straight, right angled or flexible) is based on the size and
position of the tumor. The 2.2-mm probe is used for peripheral,
smaller tumors through the fiber optic bronchoscope. The 5-mm
probe is used for larger, central tumors. The large rigid bronchoscope
allows a small suction catheter to be placed to remove blood and
secretions throughout the procedure.
Direct intrathoracic cryosurgery
The tumor should be precisely located, its size measured, and
its relation to vital structures documented. Prior to cryoprobe
insertion, needle aspiration is performed to confirm the
position of major blood vessels. The cryoprobe is inserted into
the tumor mass and the freezing continued until the iceball is
large enough to cover the tumor and a 5 to 10 mm margin of
normal lung tissue around the tumor. Two cycles of freeze/thaw
are often performed. For larger tumors, multiple cryoprobes
are applied with the aim to destroy all macroscopically visible
tumors. Necrotic tissue that formed intra-operatively is removed
mechanically. A layer of necrotic material covering the free
margin of healthy-appearing lung tissue is left in situ in order to
minimize the risk of air-leak ( 11). Another technique used for direct cryosurgery is that used
home-made liquid nitrogen fixation device to perform for the
lung cancer under the direct vision thoracotomy ( 12, 13). Percutaneous cryosurgery
Cryoablation is performed under local or general anesthesia
( 15-17). In the early stage of the practice, a 21-gauge guide
needle is inserted into the center of the targeted tumors under
CT guidance, and when it is in the optimal position, a stainlesssteel
sheath for the cryoprobe, consisting of an inner guiding
sheath and an external sheath, is inserted over the needle. The
external sheath for a 2-mm-diameter cryoprobe has inner and
outer diameters of 2 and 3 mm, respectively, and for a 3-mm
cryoprobe, these are 3 and 4 mm, respectively. After the inner
sheath is removed, either a 2- or 3-mm cryoprobe is inserted
through the external sheath, which is 180 mm long, equivalent
to the length of the cryoprobe, and therefore the cryoprobe tip is
located at the end of the sheath ( 16). However, with the progress
of technology and experience, this procedure has been simplified
instead that the direct insertion of the probe has been applied. Under the CT guidance, the cryoprobe is inserted into the
targeted tumor directly. The cryoprobe uses high-pressure argon
and helium gas for freezing and thawing, respectively, on the
basis of the Joule-Thompson principle. Cryoablation consists
of 2 cycles of 5 minutes of freezing (cooled to around –165 ˚C)
followed by slow thawing up to 20 °C and then a third cycle of
10 minutes of freezing followed by thawing. The air in the lung
can interfere with the creation of iceball. When the cryoprobe
is inserted into normal pulmonary parenchyma, initial freezing
can make an iceball of 1 cm in diameter only because the air prevents conduction of low temperatures and there is not
enough water in the parenchyma. However, after thawing, the
massive intra-alveolar hemorrhage excludes the air and results
in a larger iceball that forms in the following freezing. Therefore,
three freeze-thaw cycles need to be performed to make an iceball
of 2.5 to 3.0 cm in diameter. The 2- or 3-mm diameter cryoprobe
can freeze an area of 2 cm and 3 cm in diameter, respectively, and
4 cm long after 3 cycles of freezing and thawing. Therefore, for
tumors smaller than 2 cm, only 1 cryoprobe is usually inserted,
and for tumors of 2 cm and more in size, 2 or more cryoprobes
are used simultaneously to ensure a freezing margin. For those
with metastases in both lungs, cryotherapy should be performed
separately in an interval of 7 days with each one side for the
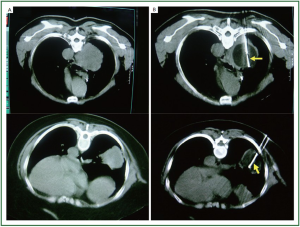
safety ( Figure 1) ( 15). After procedure, the probe is gently withdrawn. Intraoperative
thoracentesis will be performed if pneumothorax or
haemopneumothorax is evident by CT scans. Antibiotics and
haemostatics if necessary should be administered for 3 days
after cryotherapy. While in the old days, after the cryoprobe is
removed, fibrin glue is infused into the outer sheath, the outer
sheath is removed while the inner sheath is used to push the
coagulated fibrin into the cryoprobe track to reduce the risk of
bleeding and pneumothorax ( 15, 17). |
|
Clinical data
Endobronchial cryosurgery
Endobronchial cryosurgery for the endobronchial tumors was
first reported in 1986 and has since been used in over 1,000
patients, and has been proved to be a safe method for palliation
of malignancies causing airway obstruction.
In 2004, Maiwand et al. ( 10) reported that a total of 521
consecutive patients with advanced obstructive tracheobronchial malignant tumors underwent cryosurgery. The tumor was
shrunk or eradicated and lung atelectasis was improved.
Hemoptysis, cough, dyspnoea and chest pain were improved
by at least one class in 76.4%, 69.0%, 59.25% and 42.6% of
symptomatic patients respectively, and improvement in one or
more symptoms was demonstrated in 86% of patients. Median
survival was 8.2 months and 1- and 2-year survival was 38.4%
and 15.9%, respectively. Asimakopoulou et al. ( 11) compared the efficacy between
at least two sessions and one session of endobronchial
cryosurgery. Group A including 172 patients with at least two
sessions of endobronchial cryosurgery was compared with
group B including 157 patients with one session of cryosurgery
for primary or metastatic obstructive lung carcinoma. Results
showed that symptoms of dyspnea, cough, and hemoptysis
were significantly reduced in both groups after cryosurgery,
although group A benefited more than group B. Lung function
was improved significantly in group A. The mean Karnofsky
performance score had a similar increase in both groups. The
mean survival was 15 months (median, 11 months) for group
A and 8.3 months (median, 6 months) for group B. Univariate
regression analysis showed that no particular patient or tumor
characteristic was associated with reduction of symptoms.
Patients who had cryosurgery and external beam radiotherapy
showed longer survival. Females and patients with stage IIIa and
IIIb tumors achieved significantly improved Karnofsky scores. Yu et al. ( 18) investigated the effect of endobronchial
cryosurgery in 92 patients with central bronchial carcinoma
using CO2 as the cryogen. Tumor complete remission (CR)
achieved in 51 (55.4%) patients and partial remission (PR) in
31 (37.7%) patients. Cough, hemoptysis, dyspnoea, and chest
pain were improved in 73.9%, 98.0%, 75.0%, and 50.0% of the
patients. Obstructive pneumonia was controlled in 87.2% of the
patients. Wang ( 19) also used CO 2 as the cryogen for cryosurgery
under guidance of bronchoscope for endobronchial
malignancies. His experience showed that cryoextraction could
be used for large tumors, and destruction was recommended
for relatively superficial lesions. The clinical outcome and
complication of cryosurgery were mainly attributed to the
technique and experience of surgeon, patient status and tumor
characteristics. Nevetheless, cryosurgery is one of the safer and
effective therapies for endobronchial obstructed lesions. Direct cryosurgery
Liu ( 12) first used home-made liquid nitrogen fixation device to
perform the lung cancer under the direct vision thoracotomy. The
results showed 9 (24.3%) out of 37 cases of primary pulmonary
cancer survived more that 5 years. Others used similar device to
have direct cryotherapy under open thoracotomy ( 13, 20, 21).
Chen et al. ( 13) treated the patients with primary and metastatic
lung cancer in 34 cases. Most of the patients (32/34) turned to
be operable after intraoperative cryosurgery. The 1-, 2-, and 3-year
survival were 90.9%, 47.3%, and 32.5%, compared with that of
76.4%. 45.0%, and 28.0%, respectively, in surgical resection only. Maiwand et al. ( 10) reported direct cryosurgery on lung
cancer which was performed on 15 patients at exploratory
thoracotomy. The intraoperative findings of no possibility of
resection for tumor led to the decision not to perform lung
resection, instead, to receive direct cryosurgery. There were no
post-operative complications attributable to the application of
direct cryosurgery. In particular, there were no cases of prolonged
air-leak or pneumothorax post-operatively. The results showed an
improvement in the respiratory function over an average followup
period of 9 months. Performance status and symptoms such
as cough, dyspnoea and hemoptysis were shown improvement in
77.8%, 66.7%, and 100% of symptomatic patients, respectively.
Measurable reduction in tumor mass was recorded in three of
the fifteen patients. The median survival from the date of surgery
was 11.6 (6.8 to 18.2) months, range 1 to 84 months. Oneyear
survival was 50% with 25% and 6% surviving 2 and 5 years
respectively. Zhuang et al. ( 14) investigated the efficacy and the safety
of intraoperative cryoablation in 15 patients with inoperable
lung cancer. The results showed that cough, hemoptysis, and
dyspnoea were improved significantly after cryoablation and the
average survival time was 11.6 months. We also performed intrathoraxic cryotherapy for inoperable
lung cancer in 36 cases before 2006. The results showed a similar
benefit with the percutaneous modality ( 22). Percutaneous cryosurgery
Kawamura et al. ( 17) reported that 35 small pulmonary
malignant tumors in 20 nonsurgical patients were given
percutaneous cryoablation under CT guidance with local
anesthesia. Results showed that local recurrence of 7 (20%)
tumors in 7 (35%) patients during a 9 to 28 months (median,
21 months) follow-up period. One-year survival according to the
Kaplan-Meier method was 89.4%. Choe et al. ( 23) performed percutaneous cryotherapy
in 9 of 76 procedures in 65 patients with relative small lung
cancer (about 2 cm), others were performed by radiofrequency
ablation with tumor around 4 cm. The overall median survival
was 20.8±4.7 months with 1-, 2-, and 3-year survival rates of
all patients being 67%, 46% and 27%, respectively. The results
showed that cryotherapy was safe with less complications
compared with RFA. Since 2000 when Wu in Shanghai performed the first CTguided
percutaneous cryosurgery for lung cancer in the world ( 24),
percutaneous cryosurgery for lung cancer has been widely performed in China ( 15, 16, 20, 21, 25-34). Niu and his colleagues ( 15) from Fuda Cancer Hospital,
Guangzhou, reported the results of a total of 840 patients
with non-small cell lung cancer who underwent percutaneous
cryoablation in Fuda Cancer Hospital Guangzhou, China. Based
on the TNM staging, there were 122 patients with stage IIa,
462 with IIb, 160 with IIIa, 64 with IIIb and 32 with IV. A total
of 1,174 procedures of cryoablation were performed for 840
patients with lung cancer. There were 140 and 66 patients who
underwent additional single and two sessions of cryoablation
procedure, respectively, for recurrent tumors in the lungs.
There were 62 patients who underwent additional session of
cryoablation for liver metastases. After cryoablation, the size of
the lesions increased initially, which was corresponding to the
freezing margin exceeding 1 cm beyond the limit of the tumor.
The cryotreated lesions then appeared shrinking or cavitated on
CT images. During the follow-up, complete remission (CR) was
observed in 86 patients (14.4%), partial remission (PR) in
588 patients (70.0%). However, the tumor recurred in 47.2%
of the patients during a median follow-up of 34 months (range,
4 to 63 months), in the lungs, liver, brain, and bone. The
recurrence at cryosite accounted 28.3% of cases. During the
follow-up, the median survival of all patients was 23 months
(range, 5-61 months) with 1-, 2-, 3-, 4-, and 5-year overall
survival of 68%, 52%, 34%, 26% and 17%, respectively. Zhou
et al. ( 35) in the same hospital observed therapeutic effects of
cryosurgery combined iodine-125 seeds implantation in 140
patients with advanced lung cancer. However, the combination
treatment did not show much better result than the whole
group above mentioned. After 6 postoperative months the
patients had CR of 16.8%, PR of 70.1%, stable disease (SD)
of 7.4%, and progressive disease (PD) of 5.7%. The half-year
and one-year survival rates were 94.3% and 65.7% respectively.
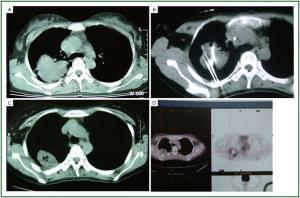
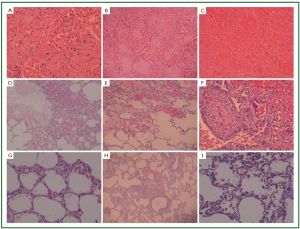
Figure 2, 3 showed two patients with complete response as
proven by histology. Wang et al. ( 16) from PLA General Navy Hospital, Beijing,
reported initial experience with CT-guided percutaneous
cryotherapy of primary and metastatic lung malignancies who
were not surgical candidates in 187 patients. Ice formation was
identified at CT as reduced attenuation values (in Hounsfield
units) within soft-tissue masses, and tumor size and location
were independent predictors of tumor coverage by low attenuating ice. The overall rate of pneumothorax was 12% (22 of
187 patients), and other side effects appeared to be self-limited. Chen et al. ( 27) found that all the tumors shrank at one
post-cryoablation month compared with one week before
cryoablation, with the average size of 5.61±3.13 mm reduced to
5.15±3.00 mm, the recovery rate of 10.29% and total effective
rate of 98.52%. Feng et al. ( 25) from PLA General Navy Hospital, Beijing,
compared the efficecy of cryosurgery combined with or without
chemotherapy in 253 patients with advanced non-small cell lung
cancer in a randomized study. The results demonstrated the overall
survival in combination therapy group was 15.10±3.84 months
compared with 10.08±1.02 months in cryosurgery group, while
there was no difference between ice coverage to tumor in the two
groups. Zhang et al. ( 36) from Hebei Province reported 62 patients
with uncontrolled nonsmall cell lung cancer after common
radiotherapy and/or chemotherapy who were re-treated
with therapeutic alliance of cryotherapy and interventional
chemotherapy, with 1-year survival of 80.1%. Du et al. ( 20) from Beijing compared cryotherapy and
surgical resection in 26 and 18 patients, respectively, and
results showed that the cryotherapy group had less local
recurrence and distal metastases with 1-year survival (75%)
and 3-year survival (33.5%) of cryoablation obviously
higher than that of resection (58.3% and 0%, respectively)
though this study was not randomized and groups were not
well matched. A meta analysis assessed the survival rate and
quality of life of patients with intermediate and advanced
non-small cell lung cancer after cryoablation. Forty-four
papers on treatment of intermediate and advanced NSCLC
with argon-helium cryoablation were searched from “lung
cancer” and “argon-helium cyroablation”. It is suggested that
cyroablation can improve the quality of life in patients with intermediate and advanced NSCLC, while combination with
radiotherapy and chemotherapy does not show any clinical
advantages over cryoablation alone and even decreases the
quality of life of such patients ( 30). Luo et al. ( 31) of Meitan General Hospital, Beijing,
reported the results of the 139 patients with unresectable nonsmall
cell lung cancer patients confirmed by pathology and
with follow-up from July 2006 to July 2009. Combination of
multiple minimally invasive treatments was selected according
to the blood supply, size and location of the lesion. Among
the 139 cases, 102 cases of primary and 37 cases of metastasis
to mediastinum, lung and chest wall, 71 cases with tumors
abundant in blood supply were treated with the combination
of superselective target artery chemotherapy, percutaneous
cryoablation and radiochemotherapy with seeds implantation;
48 cases with tumors poor in blood supply were used single
percutaneous cryoablation; the other 20 cases with tumors
poor in blood supply used the combination of cryoablation and
radiochemotheraoy with seeds implantation. The KPS score
increased after the treatment. During the follow-up of 3 years,
the results showed CR in 44 cases which were all treated with
either cryoablation or cryoablation plus radiochemotherapy, PR
in 87 cases, and the efficacy was 94.2% with median survival of
19 months (mean 16±1.5 months), and 1- and 2-year survival of
71.2% and 30.2%, respectively. In 2007, Hu et al. ( 26) from Dongfang Hospital, Beijing
University of Chinese Medicine, observed the clinical effect
of the combined therapy with argon-based cryosurgery and
Chinese herbal medicine in treating 57 NSCLC patients. The
treatment was successful in all patients with mild adverse
reactions. The effective rate was 83.8%, 79.6%, and 77.3% at 3, 6,
and 12 months after treatment with median survival of 9 months,
and the 1- and 2-year survival of 46.67% and 36.36%, respectively. These results show that the percutaneous cryotherapy with
or without other modalities is a safe and effective option for the
treatment of lung cancer.
|
|
Experimental study
We evaluated ( 37) lung necrosis by CT-scan and histology
in a porcine model using different freeze-thaw cycles during
percutaneous cryosurgery under CT guidance. Three cryoprobes
were inserted into both the left and right lung for each pig,
respectively. For the left lung cryoablation was performed by two
cycles of freezing with each 10 minutes followed by 5-minute
thawing, while for the right lung, the cycles were the same as
the left lung but with each 5-minute freezing and plus another
(third) cycle of 10-minute freezing and 5-minute thawing.
The cryolesional samples were taken at 4-hour, 3 and 7 days
postoperatively. Our results showed the ice-ball grew gradually
in relation to the increase in time and cycles. The size of the
cryolesion became larger than the ice-ball during cryosurgery,
regardless of 2 or 3 freeze-thaw cycles were performed. The area
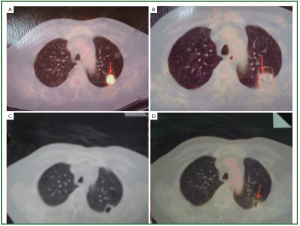
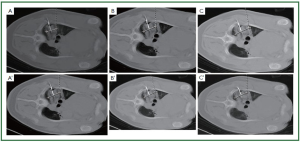
of necrosis gradually increased as time increased ( Figures 4, 5, 6).
It is suggested that three freeze-thaw cycles are necessary for the
complete cryoablation of lung parenchyma, and “1-cm safe rim”
may be not necessary during lung cryosurgery in order to avoid
harming the organ and tissue close to the cancer. Lu et al. ( 34) have investigated tumor marker changes of
48 patients with non-small-cell lung carcinoma treated by
cryosurgery only. The serum levels of 12 tumor makers were
compared between one week before and 1 month after treatment
by the multi-tumor marker protein chip diagnostic system.
Results demonstrated the serum levels of CEA, CA199, CA125,
CAl53, CA242, and Ferritin were significant decreased after
treatment. It is suggested that this system could be used as
effective evaluation of non-small-cell lung cancer patients after
cryotherapy. Some in vitro studies suggest cryotherapy can stimulate the
immune system to trigger a specific anti-tumor effect in human
lung cancer cells ( 38-40). Zhou et al. ( 38) found the levels of
soluble interleukin-2 receptor (SIL2-R), IL-6 and lymphocyte
transformation rate (LTR) were all significantly increased
after freeze of lung cancer cells. The supernatant of frozen cells
could remarkably inhibit the proliferation of the autologous
lung cancer cells, indicating that cryotherapy could provoke
the antitumor immunity. The bone marrow-derived dendritic
cells (DCs) could efficiently process and present the antigens
of freeze-thawing treated cancer cell, and subsequently activate
CTLs or NK cells and induce cancer cell apoptosis. Secretion of
IL-12 by DCs was enchanced when cultured with cryo-treated
lung cancer cells ( 41-43). Cryo-treated lung cancer cells could
activate DCs to secrete IL-12 as well as DCs maturity, thus to kill
cancer cells specifically ( 39). Li et al. ( 42) found tumor infiltrating lymphocytes (TIL)
from lung cancer had no change after the treatment of liquid
nitrogen freezing and thawing, suggesting immunologic property
and cytolytic activity of TIL can be well kept after liquid nitrogen
freezing. Other studies have been focused on the enhancement of
cryotherapy combined with other treatments. In a series of
investigations on a lung cancer animal model, Forest et al.
( 43, 44) founded the injection of Vinorelbine ditartrate at
15 days after cryotherpay induced much more amount of
necrosis in tumors and much more important in the T/C ratio.
The combination of cryotherapy and chemotherapy could
enhance both necrosis and apoptosis of the tumor. Others
have investigated the immunomodulators on the enhanced
cryotherapy. Redondo et al. ( 45) documented that cryotherapy
of the tumor with topical administration of imiquimod induced
potent antitumor immune response and protected 60% of
the animals against tumor rechallenges. den Brock et al. ( 46)
demonstrated that local recurrence at the ablated site was
reduced from 30% to 0% and cryoablation-induced immune
response was enhanced when in combination with CpGoligodeoxynucleotide
administration. These results confirm that
cryotherapy can enhance the uptake of tumor antigens by the
dendritic cells. However, there is no evidence so far this will be
the same in lung cancer. |
|
Discussion
Patients with advanced non-small-cell lung cancer have a poor
outcome. No clear-cut consensus regarding the management of
this disease has been established worldwide. Despite advances
in treatment, the overall survival has not improved substantially
during the past 30 years. The 1- and 2-year survival rate has not
exceeded 20% and 10%, respectively ( 47). There are few options available when lung cancer is considered
unresectable. Cryosurgery is one of the promising techniques.
Based on the experience with endobronchial and direct
cryoablation for lung cancer ( 10, 11) and the successful results of
percutaneous cryoablation of liver and prostate carcinoma ( 7, 9),
percutaneous cryoablation technique for the treatment of lung
cancer has been more and more widely applied. Endobronchial and direct cryosurgery
The advantages of endobronchial cryosurgery are proved
effective and with minimal complications. It is relatively easy
to use and economical in comparison with other techniques.
Patients tolerate the procedure very well and show a significant
improvement in symptoms at the end of the procedure. The use
of a general anaesthetic has the advantage that it allows greater
head and neck mobility and makes a patient more relaxed. General
anaesthetic, however, may carry some risks in frail patients.
Complications with endobronchial cryotherapy seem to be
acceptable. In the report of Maiwand et al. ( 10), 9% of the patients
were with post-operative complications in which 21 cases with
hemoptysis (4%), 12 cases with post-operative atrial fibrillation
(2%) and 16 patients with respiratory distress and poor gas
exchange that eventually resolved (3%). In addition, 7 (1.2%)
patients died of respiratory failure. However others reported no
serious complicatons ( 16-18). The reasons for this difference are
not clearly defined with the possible assciation with the patients’
characteristics and surgeon’s experience. The direct cryosurgery, which can be performed under
either open thoracotomy or thoracoscope, provides a precise
location and management of the tumor. The recurrence has
been significantly decreased at the edge of resection when the
cryoablation is employed for this area ( 13, 20, 21). It is safe also
to perform the cryotherapy under open thoracotomy. There
were no significant post-operative complications attributable to
the application of direct cryosurgery including pneumothorax
( 10-14). However, it is more invasive than the following
percutaneous pathway. Percutaneous cryosurgery
Efficacy of percutaneous cryosurgery
The efficacy of cryoablation for lung cancer is much better than that of chemotherapy with or without radiation in recent
reports. Our study showed a superior benefit compared with
the above results in 840 patients with non-small cell lung cancer.
The median survival of all patients was 5 to 61 months (mean 23).
Overall 1-, 2-, 3-, 4-, and 5-year survival were 68%, 52%, 34%, 26%
and 21%, respectively ( 15). In our late experience, i.e. from 2008,
the 1-year overall survival was 66% for the whole 144 patients and
58% for stage IIIB+IV lung cancer. Two-year overall survival was
48% for the whole and 64% for the NSCLC patients of stage
IIIB+IV ( 48). Other investigations afore described have also
comfirmed the efficacy of cryosurgery for lung cancer ( 20, 31, 35). Safety of percutaneous cryosurgery
During the percutaneous cryoablation for lung cancer,
pneumothorax is a very common complication, which was seen
in 25.9% in our study ( 15) and 12% in other report ( 16); pleural
effusion and hemoptysis are also common, seen in 16.2% and
22.5% of our series, respectively ( 15), and two complications
involved recurrent laryngeal nerve damage observed but the
patients regained speech within 2 months ( 10). Niu and his colleagues ( 49) from Fuda Cancer Hospital-
Guangzhou, analyzed the most common complications after
percutaneous cryoablation for advanced lung cancer. A total of
644 lung cancer patients had been treated with percutaneous
cryoablation guided by ultrasound and/or CT scan, and
showed that complications were relatively minor and generally
didn't have life-threatening consequences, and were resolved
spontaneously or with conservative management. No severe
complications such as cryoshock and renal insufficiency, as
observed during liver cryoablation ( 50, 51), were seen in this
series. The 30-day mortality was 2.6% in our study ( 15). Serious
complications included cardiac arrest and hemopneumothorax,
and thus preventative steps should be taken. Therefore, CTguided
percutaneous lung cryotherapy yielded lower procedural
morbidity. Survival analysis of percutaneous lung cryotherapy
There are few investigations on the impact of pulmonary
cryotherapy on long-term survival for the advanced lung cancer
patients. We have studied the survival analysis in 144 patients
with Cox regression model and showed that factors associated
with better survival included female gender, stage (III or IV),
previous treatment, chemotherapy, and cryotherapy followed
by chemotherapy ( 48). Li et al. ( 52) investigated the longterm
effects and risk factors of percutaneous cryosurgery for
253 patients with advanced lung cancer. In the follow-up of 6
to 55 months, the median survival time was 11.98 months and
1-, 2-year survival rate was 41.1%, 27.59%. The multivariant
analysis by Cox model revealed that the tumor staging (IIIb or
IV), tumor size (<3 cm or > 4cm), location (upper lobe or lower
lobe) and combination chemotherapy (≥2 cycles or <2 cycles) were significantly associated with prognosis of NSCLC. Choe
et al. ( 23) also found that the complete ablation for the tumors
had been significantly associated with higher survival duration
and progression free survival duration compared with the partial
ablation. It is evident that further investigations based on longterm
follow-up in randomized and controlled trials need to
declare the precise procedrue for the treatment of patients with
lung cancer. |
|
Conclusions
Percutaneous cryoablation offers an effective therapy for patients
with locally advanced non-small-cell lung cancer, without
serious complications. It is especially suitable for the treatment
of unresectable lung tumors (e.g., the cancer with multiple
nodules, large tumor and ill-located tumor) and for the cancer
patients with co-morbidity conditions considered to be poor
surgical candidates. The therapeutic efficacy of the procedure is
preponderate over that of routine chemotherapy and radiation.
In vitro studies have shown cryotherapy of lung cancer cells can
improve the immune system to trigger the specific anti-tumor
response. However, this study is still a preliminary one. In the
future, comparative studies between this modality and wedge
resection, stereotactic radiation or other therapies should be
conducted to further determine the efficacy and role of this
novel approach for the treatment of lung cancer. In addition,
more attention needs to be put on the immunomodulators that
enhance the cryoimmunology. Nevertheless, according to the
current data, percutaneous cryoablation, a feasible and miniinvasive
technique, has demonstrated an encouraging efficacy in
the treatment of advanced non-small-cell lung cancer.
|
|
Acknowledgements
Disclosure: The authors declare no conflict of interest.
|
|
References
- Yang P. Epidemiology of lung cancer prognosis: quantity and quality of life.
Methods Mol Biol 2009;471:469-86.
- Stracci F. Cancer screenings, diagnostic technology evolution, and cancer
control. Methods Mol Biol 2009;471:107-36.
- Vogl TJ, Straub R, Lehnert T, et al. Percutaneous thermoablation of
pulmonary metastases. Experience with the application of laser-induced
thermotherapy (LITT) and radiofrequency ablation (RFA), and a literature
review. Rofo 2004;176:1658-66.
- Simon CJ, Dupuy DE. Current role of image-guided ablative therapies in lung
cancer. Expert Rev Anticancer Ther 2005;5:657-66.
- Gillams A. Lung tumour ablation - where are we now? Cancer Imaging
2008;8:116-7.
- Roy AM, Bent C, Fotheringham T. Radiofrequency ablation of lung lesions: practical applications and tips. Curr Probl Diagn Radiol 2009;38:44-52.
- Xu KC, Niu LZ, He WB, et al. Percutaneous cryoablation in combination
with ethanol injection for unresectable hepatocellular carcinoma. World J
Gastroenterol 2003;9:2686-9.
- Mouraviev V, Polascik TJ. Update on cryotherapy for prostate cancer in
2006. Curr Opin Urol 2006;16:152-6.
- Xu KC, Niu LZ. Cryosurgery for Cancer. In: Lung Cancer. Niu LZ, Xu
KC, eds. Shanghai: Shanghai Science and Technology Education Press,
2007:106-22.
- Maiwand MO, Asimakopoulos G. Cryosurgery for lung cancer: Clinical
results and technical aspects, Technol. Cancer Res Treat 2004;3:143-50.
- Asimakopoulos G, Beeson J, Evans J, et al. Cryosurgery for malignant
endobronchial tumors: analysis of outcome. Chest 2005;127:2007-14.
- Liu P. Pulmonary cryosurgery. Low Temp Med 1985;11:84.
- Chen WP, Cheng YQ, Zhen Q. Clinical evaluation of intraoperative
cryosurgery with liquid nitrogen for the lung cancer. Ai Zheng 1990;9:387-8.
- Zhuang CW, Zhang ZM, Weng XQ, et al. Intraoperative Ar-He targeted
cryoablation for inoperable lung cancer, Lin Chuang Jun Yi Za Zhi
2008;36:57-8.
- Niu LZ, Xu KC, He WB, et al. Percutaneous Cryoablation for patients
with advanced non-small cell lung cancer. Technol Cancer Res Treat
2007;6:451-2.
- Wang H, Littrup PJ, Duan Y, et al. Thoracic masses treated with
percutaneous cryotherapy: initial experience with more than 200
procedures. Radiology 2005;235:289-98.
- Kawamura M, Izumi Y, Tsukada N, et al. Percutaneous cryoablation of small
pulmonary malignant tumors under computed tomographic guidance with
local anesthesia for nonsurgical candidates, J Thorac Cardiovasc Surg 2006;
131:1007-13.
- Yu XY, Tang X, Liu HY, et al. Cryosurgery under bronchoscope guidance
for the patients with central bronchial carcinoma. Yi Shi Jin Xiu Za Zhi
(Neike) 2004;27:37-8.
- Wang HW. Endobronchial cryosurgery under guidance of bronchoscope.
Zhongguo Zuzhi Gongcheng Yan Jiu yu Lin Chuang Kang Fu
2008;12:5001-6.
- Du XS, Chen YF, Han XD, et al. Cryotherapy and resection for primary
peripheral lung cancer. Zhonghua Xiong Xin Xue Guan Wai Ke Za Zhi
2003;19:24.
- Zhang BJ, Yang RS, Liu P, et al. Clinical study on cryosurgery in lung
cancer. Journal of Cancer Prevention and Treatment 2004;11:728-9.
- Niu LZ, He WB, He YS, et al. Clinical evaluation of cryoablaton for 508
patients with lung cancer. Zhongguo Jiao Tong Yi Xue Za Zhi 2006;20:29-30.
- Choe YH, Kim SR, Lee KS, et al. The use of PTC and RFA as treatment
alternatives with low procedural morbidity in non-small cell lung cancer.
Eur J Cancer 2009;45:1773-9.
- Zhang JR. Argon-Helium cryoablation in the treatment for cancer.
Zhongguo Zhong Liu 2007;16:335-7.
- Feng HS, Nie ZS, Duan YY, et al. Clinical study of percutaneous cryosurgery
combined with chemotherapy in treatment for 253 cases with advanced nonsmall
cell lung cancer. Zhongguo Zhong Liu 2007;16:898-901.
- Hu KW, Li QW, Zuo MH, et al. Clinical observation on the combined
treatment of 57 cases of non-small cell lung cancer using argon-helium
cryosurgery and Chinese herbal medicine. Chin J Integr Med 2007;13:224-7.
- Chen B, Xu J, Cao JM, et al. Therapeutic assessment of cryoablation
for the treatment of lung cancer. Journal of Interventional Radiology
2009;18:510-40.
- He ES, Luo YC, Luo FR. Clinical observation of cryotherapy for advanced
lung cancer under MRI guidance. Wei Chuang Yi Xue 2009;4:251-2.
- Wu LH, Zhao DY, Fu YM, et al. Cryotherapy of lung cancer under MRI
guidance system. Zhongguo Wei Chuang Wai Ke Za Zhi 2009;9:437-8.
- Du XF, Han BS, Li TZ. Effect of argon-helium cryoablation on intermediate
and advanced non-small cell lung cancer: A meta-analysis. J. Chin. PLA
Postgrad. Med. Sch 2010;31:714-7.
- Luo L, Wang H, Ma H, et al. TACE with Ar-He cryosurgery combined
minimal invasive technique for the treatment of primary NSCLC in 139
cases. Zhongguo Fei Ai Za Zhi 2010;13:60-3.
- Bu J, Quan XY, Liang W, et al. Imaging evaluation of lung cancer after
cryoablation under CT guidance. Shi Yong Yi Xue Za Zhi 2010;26:1601-3.
- Liu YX, Liu DX, Huang HZ, et al. Low-dose CT-guided percutanous
minimal invasive cryosurgical treatment of lung tumors. Modern Hosipital
2010;10:13-7.
- Lu N, He JH, Huang JG, et al. The effects of multi-tumor marker in nonsmall
lung carcinoma treated by argon-helium cryosurgery system. China
Medical Engineering 2010;3:28-30.
- Zhou H, Niu L, Zhou L, et al. Cryosurgery combined with Iodine-125 seed
implantation in the treatment of unresectable lung cancer. Zhongguo Fei Ai
Za Zhi 2008;11:780-3.
- Zhang FT, Li XL, Li HJ, et al. Clinical analysis of the therapy for recurrent
and intractable non-small cell lung cancer with combination of cryoablation
and intervention. Journal of Interventional Radiology 2007;16:759-61.
- Niu L, Wang J, Qiu D, et al. Imaging and pathological features of
percutaneous cryosurgery on normal lung evaluated in a porcine mode.
Zhongguo Fei Ai Za Zhi 2010;13:676-80.
- Zhou P, Lu Z, Zhang G. A study on immunity enhancement of frozen
human lung cancer cells in vitro. Zhongguo Fei Ai Za Zhi 1999;2:32-4.
- Li YQ, Feng HS, Huang YZ, et al. Changes of peripheral mononuclear cell
immunologic function caused by jung cancer cell treated with Ar-He freeze.
ZhongguoZhongLiu 2007;16:895-7.
- Wang, HW. Immunomodulatory effects of cryolytic lung cancer cells on
bone marrow-derived dendritic cells. Zhongguo Zu Zhi Gong Cheng Yan
Jiu yu Lin Chuang Kang Fu 2009;13:3560-4.
- Feng HS, Huang YZ, Duan YY, et al. Enhancement of dendritic cell induced
antitumor effect by lung cancer cell after cryotreatment. Sheng Wu Yi Xue
Gong Cheng Yan Jiu, 2005;24:115-6.
- Li GZ, Guo BQ, Kang B, et al. Observation of antitumor activity on tumor
infiltrating lymphocytes from lung cancer in liquid nitrogen cryopreservation.
WeifangYixueyuanXuebao 2000;22:24-5.
- Forest V, Peoc'h M, Campos L, et al. Effects of cryotherapy or
chemotherapy on apoptosis in a non-small-cell lung cancer xenografted
into SCID mice. Cryobiology 2005;50:29-37.
- Forest V, Peoc'h M, Ardiet C, et al. In vivo cryochemotherapy of a human
lung cancer model. Cryobiology 2005;51:92-101.
- Redondo P, del Olmo J, López-Diaz de Cerio A, et al. Imiquimod enhances
the systemic immunity attained by local cryosurgery destruction of
melanoma lesions. J Invest Dermatol 2007;127:1673-80.
- den Brok MH, Sutmuller RP, Nierkens S, et al. Synergy between in situ
cryoablation and TLR9 stimulation results in a highly effective in vivo
dendritic cell vaccine. Cancer Res 2006;66:7285-92.
- Morgensztern D, Waqar S, Subramanian J, et al. Improving survival for
stage IV non-small cell lung cancer: a surveillance, epidemiology, and end
results survey from 1990 to 2005. J Thorac Oncol 2009;4:1524-9.
- Niu LZ, Xu KQ, Zhou L, et al. Impact of percutaneous cryotherapy on
survival of advanced lung cancer with or without chemotherapy, Low Temp
Med 2010;2:41-6.
- Niu L, Wang J, Zhou L, et al. Complications of cryoablation in 644 lung
cancer patients and its treatment. Zhongguo Fei Ai Za Zhi 2010;13:832-4.
- Pearson AS, Izzo F, Fleming RY, et al. Intraoperative radiofrequency ablation
or cryoablation for hepatic malignancies. Am J Surg 1999;178:592-9.
- Seifert JK, Morris DL. World survey on the complications of hepatic and
prostate cryotherapy. World J Surg 1999;23:109-13.
- Li YQ, Feng HS, Nie ZS, et al. The long-term effects and risk factors analysis
in 253 cases advanced non-small cell lung cancer treated with percutaneous
cryosurgery. Lin Chuang Zhong Liu Xue Za Zhi 2010;15:346-9.
Cite this article as: Niu L, Xu K, Mu F. Cryosurgery for
Lung Cancer. J Thorac Dis 2012;4(4):408-419. doi:
10.3978/j.issn.2072-1439.2012.07.13
|