
Charlson comorbidity index as a predictor of short-term outcomes after pulmonary resection
Introduction
Lung cancer is the leading cause of cancer death worldwide, accounting, for approximately 1.4 million deaths annually (1). The most effective treatment for early-stage lung cancer is surgical resection. However, only about 20% to 30% of patients are potential candidates for surgical resection because of the stage at which the disease is diagnosed or because of comorbid conditions (2). Comorbidity has been associated with overall and disease-specific outcomes in various cancers. Compared with patients with many other types of cancer, patients with lung cancer are more likely to harbor substantial comorbidity because of their advanced age at diagnosis and a strong association with cigarette smoking (3). All these comorbidities can have deleterious effects on the diagnostic procedures and, moreover, on the treatment possibilities and thus must be carefully explored. The Charlson comorbidity index (CCI) have been used to assess the comorbidity risk associated with several conditions. CCI is closely related to postoperative complications (4).
The objective of this retrospective study is to evaluate the impact of the CCI on short-term outcomes after pulmonary resection, to determine the cutoff point of CCI with guiding significance for prognosis.
We present the following article in accordance with the STARD reporting checklist (available at http://dx.doi.org/10.21037/jtd-20-2264).
Methods
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the institutional ethics board of China-Japan Friendship Hospital (No. 2018-13-K08) and individual consent for this retrospective analysis was waived.
Data collection
We retrospectively analyzed 1,309 patients who underwent pulmonary resection in China-Japan Friendship Hospital between January 2010 and January 2018. Perioperative data were collected for all patients, including age, sex, smoking history, American Society of Anesthesiologists (ASA) classification, CCI, surgical procedure, anesthesia time, operation time/one-lung ventilation time, blood loss, histology, complications (air leakage more than 1 week, pleural effusion needs re-thoracentesis, pulmonary infection (fever and culture positive), atrial fibrillation, re-thoracotomy, postoperative blood transfusion, pulmonary embolism, pneumothorax, chylothorax (whether somatostatin therapy is effective), atelectasis and/or sputum retention requiring bronchoscopy, wound sepsis and bronchopleural fistula), poor outcomes, 30-day mortality, and postoperative stay. However, since not all of these complications were irreversible or untreatable, not all of them would necessarily have precluded surgery if predicted in advance. We defined postoperative death or respiratory failure as poor outcomes. Poor outcomes were used to identify patients who should ideally be identified as high-risk patients before surgery.
CCI scoring
Comorbidity was defined as the presence of one or more additional conditions existing simultaneously, independently or not (with or without a causal effect) with a disease that was considered primary. In the CCI the patient’s age was allocated to one of the age groups, each considered to be of different risk. Table 1 introduces the Charlson comorbidity index and the prevalence of comorbid conditions. The index score was further calculated the CCI calculator (5,6).
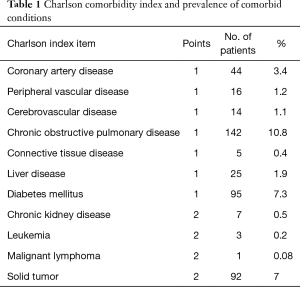
Full table
Statistical analysis
Categoric variables were expressed as percentages and evaluated with Chi square or Fisher’s exact test. Continuous data were presented as mean ± SD and were compared using the two-sample Student t test. Receiver-operating characteristic (ROC) curves were plotted to assess the predictive value of CCI for poor outcomes. All P-values were reported by 2-sided analyses, and the statistical significance level was set at less than 0.05. Statistical analysis was performed with SPSS 24.0 software (SPSS, Inc., Chicago, IL, USA).
Results
From January 2010 to January 2018, 1,309 patients underwent pulmonary resection. Demographic, clinical characteristics and operation procedures were presented in Table 2. In brief, the type of surgery performed consisted of wedge resection in 344 (33.1%) patients, segmentectomy in 38 (3.0%) patients, lobectomy in 831 (63.5%) patients, and bilobectomy in 96 (7.3%) patients. Nine hundred and six patients received the resection by video-assisted thoracoscopic surgery (VATS), the postoperative complication rate was 17.0%, 73 patients received the resection by video-assisted minithoracotomy (VAMT), the postoperative complication rate was 26.0%, 122 patients received the resection by thoracotomy, the postoperative complication rate was 27.7%. Nine hundred seventy-one (74.2%) patients were histologically diagnosed as malignant tumors, the rest 338 (25.8%) were histologically diagnosed as benign tumors. The postoperative pathology of all patients was shown in Table 3. Adenocarcinoma (AC), squamous cell carcinoma (SCC) and metastatic tumor were the top three in the malignant tumors. Tuberculosis (TB), hamartoma and inflammatory pseudotumor were the top three in the benign tumors. The proportion of each age group was shown in Table 4. There are 438 patients (33.5%) in the 51–60 age group and 425 patients (32.5%) in the 61–70 age group. Only 17 (1.2%) patients were in the age group of 81 years or older.
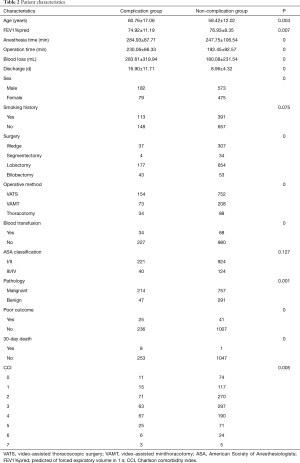
Full table
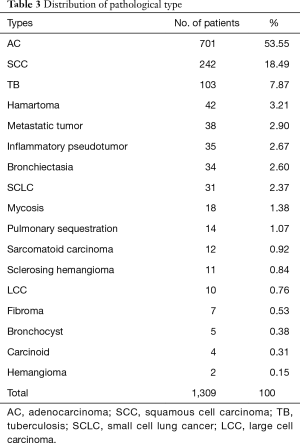
Full table
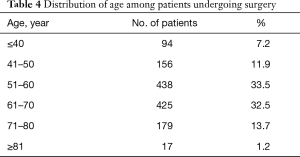
Full table
According to the occurrence of postoperative complications, they were divided into two groups. There were 261 (19.9%) patients in the complication group and 1,048 (80.1%) patients in the non-complication group. The mean age of the two groups was 60.76±17.06 and 58.42±12.02 years, respectively. In the complication group, the mean time of extubation and discharge was 12.21±10.97 days and 16.90±11.71 days. In the non-complication group, the mean time of extubation and discharge was 5.70±3.40 days (P<0.001) and 8.96±4.32 days (P<0.001). Univariate analysis identified that age (P<0.001), anesthesia time (P<0.001), operation time (P<0.001), sex (P<0.001), type of surgery (P<0.001), operative method (P=0.001), blood loss (P<0.001), intraoperative blood transfusion (P<0.001), CCI (P=0.005) were candidate risk factors for postoperative complications (Table 2). CCI (P=0.012), blood loss (P=0.015) and surgery (P<0.001) were an independent risk factors for complications in multivariate analysis (Table 5). The general rate of poor outcome was 5.0%, in the complication group and non-complication group, the rates were 9.6% and 3.9%, respectively. Nine patients died within 30 days after operation, 8 (3.1%) in the complication group and 1 (0.1%) in the non-complication group.
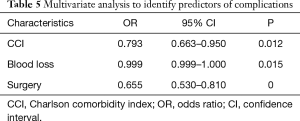
Full table
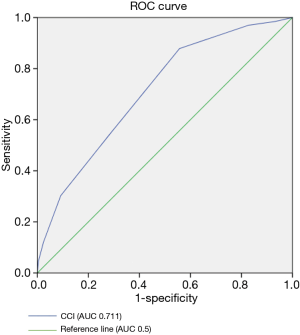
The number and proportion of complications are shown in Table 6. The more common complications were air leakage (7.5%) and atrial fibrillation (6.6%). The rare complications were bronchopleural fistula (0.3%) and pulmonary embolism (0.2%). Solid tumors, cardiorespiratory diseases, diabetes accounted for a large proportion of comorbidity. Of the 1,309 patients, 85 (6.5%) patients had a CCI of 0, 132 (10.1%) patients had a CCI of 1, 341 (26.1%) patients had a CCI of 2, 360 (27.5%) patients had a CCI of 3, 257 (19.6%) patients had a CCI of 4, 96 (7.3%) patients had a CCI of 5, 30 (2.3%) patients had a CCI of 6, 30 (0.6%) patients had a CCI of 7. To find out the relationship between poor outcomes and CCI, and whether there was a bound value with predictive value. Receiver-operating characteristic (ROC) curves were plotted in order to assess the predictive value of CCI score results for poor outcomes. Positive and negative predictive values were calculated for selected thresholds and using study estimates of the prevalence of poor outcome. Figure 1 shows the ROC curves. The diagonal line indicates an area under the curve of 0.5, equivalent to the measurement having no predictive value. The area under the curve for CCI was 0.711 (95% CI: 0.651–0.771, P<0.001). Assuming a threshold of 3 for defining poor outcomes for pulmonary resection, the sensitivity and specificity were 87.9% and 44.2%, respectively.

Full table
All patients were grouped according to their CCI score and classified into CCI ≤3 group and CCI >3 group. As shown by Table 7, there were 918 (70.1%) patients in the CCI ≤3 group and 391 (29.9%) patients in the CCI >3 group. The mean age of the two groups was 54.49±10.60 and 69.23±7.46 years, respectively. Compared with the group with CCI >3, the operation time (P=0.053) and anesthesia time (P=0.058) of the group with CCI ≤3 were shorter, the intraoperative blood loss(P=0.368) was less, the extubation time (P=0.171) and discharge time (0.067) after operation were shorter, although there was no significant difference. Most patients (87.5%) have an American Society of Anesthesiologists (ASA) classification of I/II, 91.6% in the CCI ≤3 group, and 77.7% in the CCI >3 group (P<0.001). The incidence of postoperative complications was 17.4% in CCI ≤3 group and 25.8% in CCI >3 group (P<0.001). The 30-day postoperative mortality was 0.7% in all patients, 0.5% in the CCI ≤3 group, and 1.0% in the CCI >3 group (P=0.338). The rate of poor outcome was 3.3% in the CCI ≤3 group and 9.2% in the CCI >3 group (P<0.001). In the CCI ≤3 group, the VATS proportion was 67.0%; in the CCI >3 group, the VATS proportion was 74.4%.
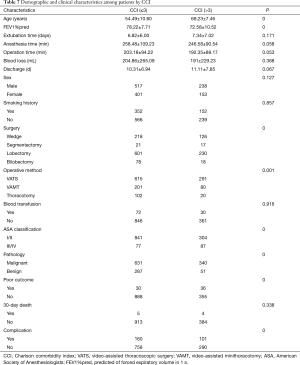
Full table
Discussion
Comorbidity was associated with increased postoperative mortality and considered to be an important prognostic factor in patients operated for cancer (7). Battafarano et al. studied 451 patients who underwent surgical resection and founded that comorbidity had a significant impact on survival (8). Powell et al. studied 10,991 patients undergoing pulmonary resection and found that there was a significant correlation between CCI and death within 90 days after the operation (9). These comorbid conditions could affect the selection of treatment options. In this retrospective analysis of 1,309 patients, we confirmed a relationship between CCI and short-term outcomes. With the increase of CCI score, postoperative complications, the rate of transfer to ICU, and death within 90 days after operation also increased. However, there remains a problem in which CCI score cutoff was of guiding significance? Wang et al. suggested that Patients with CCI ≥2 and age ≥65 years had higher perioperative mortality and death from non-cancer causes after surgery compared to patients with CCI <2 (10). The presence of comorbid conditions (CCI >1), rather than age more than 65 years, was associated with poorer survival in a large randomized trial by Asmis et al. (11). The main finding of the present study was that CCI >3 was associated with a higher incidence of postoperative complications. Our cutoff point was higher than that of previous studies. The possible reason could be that they did not include age in the calculation of the CCI score. Age and comorbidity have been associated with overall and disease-specific outcomes in various cancers. A few reports have shown, however, that advanced age is not necessarily associated with higher morbidity (11,12). Koppie et al. believed that both age and comorbidity were associated with treatment selection and survival and should, therefore, be considered (13). Wang et al. demonstrated that advancing age was a much stronger negative predictor of treatment receipt among older veterans with lung cancer than comorbidity (14). Individualized decisions that go beyond age and include comorbidity were needed to better target lung cancer treatments to older patients who might reasonably benefit. In our calculation, the age of patients in CCI is assigned to an age group, each of which is considered to have a different risk. Our study showed that patients with CCI >3 had more poor outcomes (P<0.001), but the 30-day death rate was no significant difference (P=0.338). Similarly, Battafarano showed that there was no significant trend toward higher hospital mortality with greater comorbidity (P=0.055) (8). We considered that even patients with high CCI could perform surgery safely as long as appropriate patients were selected.
In our study, the more lung tissue resected, the higher the incidence of postoperative complications. For patients with the same disease, patients with CCI ≤3 could tolerate more lung tissue resection. With the development of surgical techniques, VATS has been rapidly developed and widely applied in the world, involving almost all areas of general thoracic surgery. Compared with thoracotomy, VATS enabled a smaller incision without removing or stretching the ribs open, sparing respiratory muscles from injures and thus minimizing the loss of lung function. Patients would suffer less pain postoperatively and expectorate more easily, reducing the incidence of postoperative pulmonary infection and complications as well (15). Jeon et al. suggested that VATS lobectomy was associated with a lower incidence of pulmonary complications compared with lobectomy by thoracotomy in NSCLC patients with COPD (16). VATS lobectomy might be the preferred strategy for appropriately selected NSCLC patients with COPD. Garzon et al. believed that VATS pulmonary resection for cancer in patients with poor lung function could achieve an acceptable outcome (17). We found that the incidence of postoperative complications after thoracotomy was 1.7 times higher than that of VATS. VATS had fewer complications than other operations. Through 58 consecutive patients undergoing a VATS lobectomy for NSCLC, Nakanishi et al. demonstrated that a VATS lobectomy was a feasible and safe procedure for lung cancer in patients with a CCI score of two or more (18). In our study, the proportion of VATS used in CCI >3 group was more than that in CCI ≤3 group. For patients with CCI >3, surgeons preferred to use the most simple and quick operations. We recommended that it was very important for patients with CCI >3 to adopt the fastest way of operation and keep as much lung function as possible.
With lung cancer being far more frequent in smokers and ex-smokers, these patients often have tobacco-related illnesses, mainly cardiovascular and respiratory (19). Unfortunately, in our study, smoking history was no significant difference between the complications group (P=0.075) and the CCI group (P=0.857). We inferred that postoperative complications and CCI were more closely related to smoking index than smoking history, and then we had no smoking index data. Smoking cessation should be strongly encouraged in patients who undergo pulmonary resections, regardless of CCI.
For patients in CCI >3 group, the operation related time was shorter, hospitalization related time was longer, and the hospitalization cost was correspondingly increased. The surgeons were more rigorous in the perioperative management of such patients, hoping to reduce perioperative complications. In the research of Birim et al., the mean length of hospital stay was 14.4 days, ranging from 2 to 116. An increase of CCI score showed a slight increase in the length of hospital stay, although this was not significant (P=0.107) (4).
ASA classification was used in the anesthesiology department to evaluate the physical condition and the surgical risk of patients (20). Marret et al. (21) and Limmer et al. (22) found that the ASA classification of III and IV correlated with poor outcomes in patients receiving pulmonary resection. The higher the CCI score, the higher the ASA classification (P<0.001). Whitmore et al. demonstrated that the CCI score and ASA grade were significantly correlated, with Spearman ρ of 0.458 (P<0.001). Increasing CCI score (P=0.0032) and ASA grade (P=0.0035) were associated with an increased likelihood of complications (23).
Several limitations should be noted in this study. First, as a retrospective study, it was vulnerable to various sources of deviation that might not be identified and controlled. Second, this was a single institutional study with limited sample size and thus calls for more evidence from further prospective studies (including a larger series of patients). Future multicenter studies were needed to exploit possible predictive parameters that did not reach significance in our study, to provide better prognostic value for patients who have comorbidities.
Conclusions
CCI was associated with increased postoperative complications and considered to be an important prognostic factor in pulmonary resection patients. The main finding of the present study was that CCI >3 was associated with a poor short-term outcome. VATS pulmonary resection could be safely and effectively performed for patients with lung disease and comorbidity with satisfactory short-term outcomes. For patients with CCI >3, it was suggested that the experienced surgical team should perform pulmonary resection in the shortest time and keep the lung function as much as possible. Smoking cessation should be strongly encouraged in patients who undergo pulmonary resections. The CCI score and ASA grade were significantly correlated.
Acknowledgments
We gratefully acknowledge the role of all our colleagues, nurses and others involved in the care of these patients.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at http://dx.doi.org/10.21037/jtd-20-2264
Data Sharing Statement: Available at http://dx.doi.org/10.21037/jtd-20-2264
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd-20-2264). DL serves as an unpaid editorial board member of Journal of Thoracic Disease from Nov 2018 to Oct 2020. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the institutional ethics board of China-Japan Friendship Hospital (No. 2018-13-K08) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Parkin DM, Bray F, Ferlay J, et al. Global cancer statistics, 2002. CA Cancer J Clin 2005;55:74-108. [Crossref] [PubMed]
- Little AG, Rusch VW, Bonner JA, et al. Patterns of surgical care of lung cancer patients. Ann Thorac Surg 2005;80:2051-6; discussion 2056. [Crossref] [PubMed]
- Young RP, Hopkins RJ, Christmas T, et al. COPD prevalence is increased in lung cancer, independent of age, sex and smoking history. Eur Respir J 2009;34:380-6. [Crossref] [PubMed]
- Birim O, Maat AP, Kappetein AP, et al. Validation of the Charlson comorbidity index in patients with operated primary non-small cell lung cancer. Eur J Cardiothorac Surg 2003;23:30-4. [Crossref] [PubMed]
- Charlson M, Szatrowski TP, Peterson J, et al. Validation of a combined comorbidity index. J Clin Epidemiol 1994;47:1245-51. [Crossref] [PubMed]
- Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373-83. [Crossref] [PubMed]
- Glare P. Clinical predictors of survival in advanced cancer. J Support Oncol 2005;3:331-9. [PubMed]
- Battafarano RJ, Piccirillo JF, Meyers BF, et al. Impact of comorbidity on survival after surgical resection in patients with stage I non-small cell lung cancer. J Thorac Cardiovasc Surg 2002;123:280-7. [Crossref] [PubMed]
- Powell HA, Tata LJ, Baldwin DR, et al. Early mortality after surgical resection for lung cancer: an analysis of the English National Lung cancer audit. Thorax 2013;68:826-34. [Crossref] [PubMed]
- Wang CY, Lin YS, Tzao C, et al. Comparison of Charlson comorbidity index and Kaplan-Feinstein index in patients with stage I lung cancer after surgical resection. Eur J Cardiothorac Surg 2007;32:877-81. [Crossref] [PubMed]
- Asmis TR, Ding K, Seymour L, et al. Age and comorbidity as independent prognostic factors in the treatment of non small-cell lung cancer: a review of National Cancer Institute of Canada Clinical Trials Group trials. J Clin Oncol 2008;26:54-9. [Crossref] [PubMed]
- Pagni S, McKelvey A, Riordan C, et al. Pulmonary resection for malignancy in the elderly: is age still a risk factor? Eur J Cardiothorac Surg 1998;14:40-4; discussion 44-5. [Crossref] [PubMed]
- Koppie TM, Serio AM, Vickers AJ, et al. Age-adjusted Charlson comorbidity score is associated with treatment decisions and clinical outcomes for patients undergoing radical cystectomy for bladder cancer. Cancer 2008;112:2384-92. [Crossref] [PubMed]
- Wang S, Wong ML, Hamilton N, et al. Impact of age and comorbidity on non-small-cell lung cancer treatment in older veterans. J Clin Oncol 2012;30:1447-55. [Crossref] [PubMed]
- Oparka J, Yan TD, Ryan E, et al. Does video-assisted thoracic surgery provide a safe alternative to conventional techniques in patients with limited pulmonary function who are otherwise suitable for lung resection? Interact Cardiovasc Thorac Surg 2013;17:159-62. [Crossref] [PubMed]
- Jeon JH, Kang CH, Kim HS, et al. Video-assisted thoracoscopic lobectomy in non-small-cell lung cancer patients with chronic obstructive pulmonary disease is associated with lower pulmonary complications than open lobectomy: a propensity score-matched analysis. Eur J Cardiothorac Surg 2014;45:640-5. [Crossref] [PubMed]
- Garzon JC, Ng CS, Sihoe AD, et al. Video-assisted thoracic surgery pulmonary resection for lung cancer in patients with poor lung function. Ann Thorac Surg 2006;81:1996-2003. [Crossref] [PubMed]
- Nakanishi R, Yamashita T, Oka S. Video-assisted thoracic surgery lobectomy for non-small cell lung cancer in patients with a Charlson comorbidity index score of two or more. J Thorac Oncol 2010;5:56-61. [Crossref] [PubMed]
- Nakagawa M, Tanaka H, Tsukuma H, et al. Relationship between the duration of the preoperative smoke-free period and the incidence of postoperative pulmonary complications after pulmonary surgery. Chest 2001;120:705-10. [Crossref] [PubMed]
- Eakin JL, Bader AM. ASA physical status classification system: Is it consistent amongst providers and useful in determining need for pre-operative evaluation resources? J Clin Anesth 2017;39:73-4. [Crossref] [PubMed]
- Marret E, Miled F, Bazelly B, et al. Risk and protective factors for major complications after pneumonectomy for lung cancer. Interact Cardiovasc Thorac Surg 2010;10:936-9. [Crossref] [PubMed]
- Limmer S, Hauenschild L, Eckmann C, et al. Thoracic surgery in the elderly--co-morbidity is the limit. Interact Cardiovasc Thorac Surg 2009;8:412-416. [Crossref] [PubMed]
- Whitmore RG, Stephen JH, Vernick C, et al. ASA grade and Charlson Comorbidity Index of spinal surgery patients: correlation with complications and societal costs. Spine J 2014;14:31-8. [Crossref] [PubMed]


