
Association of early elevated cardiac troponin I concentration and longitudinal change after off-pump coronary artery bypass grafting and adverse events: a prospective cohort study
Introduction
Coronary artery bypass grafting (CABG) remains the most common cardiac surgical procedure and effective revascularization strategy for coronary artery disease (CAD) with outstanding long-term clinical outcomes, particularly for multivessel and left main disease (1). However, severe perioperative myocardial injury or cardiac dysfunction, which may be caused by ischemia-reperfusion injury, acute graft failure, and operative trauma, is associated with increased incidence of adverse events (2). Early detection of complications after CABG is important to perform timely therapeutic strategies, such as more aggressive medication, urgent re-exploration of the graft with or without redo revascularization (CABG or percutaneous intervention), and mechanical circulation assist (intra-aortic balloon pump), to reduce the risk of morbidity. Cardiac troponin is the most frequently used biomarker for detecting myocardial injury and cardiac dysfunction after CABG, and elevation of troponin levels within the first 24 hours is independently associated with worse outcomes (3,4). Several studies have been performed to investigate the association between postoperatively elevated troponin concentration and the risk of complications; nevertheless, there were some limitations. (I) A previous study assessed the troponin level on the first postoperative day (5), whereas some other studies advanced the time to less than 24 hours (3,6) or even 6–12 hours postoperatively (7); thus, the value of troponin less than 6 hours postoperatively is unclear. (II) Previous studies tended to evaluate troponin at a fixed time point; hence, data regarding the longitudinal change of troponin during the period within the first 24 hours after CABG are scarce. (III) CABG with or without cardiopulmonary bypass (CPB) and CPB time have significantly affected troponin levels (8). The current study specifically focused on off-pump CABG, whereas most former studies were performed among patients who underwent on-pump CABG. Because of the aforementioned limitations (7,9), we hypothesized that there would be a significant association between very early elevated cardiac troponin I (cTnI) concentration and its longitudinal change after off-pump CABG and adverse events. Therefore, this study aimed to investigate the association between very early cTnI concentration and its longitudinal change within 24 hours after CABG and 30-day adverse events. We present the following article in accordance with the STARD reporting checklist (available at http://dx.doi.org/10.21037/jtd-20-1691).
Methods
Study design and patients
This prospective study enrolled consecutive patients with CAD who underwent CABG from January 2019 through May 2019 at a cardiac center, Beijing Anzhen Hospital, Beijing, China. All patients received CABG in accordance with contemporary practice guidelines (1), heart team judgments, and patient preferences. Patients who underwent other concomitant cardiovascular surgery were excluded, while patients who underwent off-pump CABG and had at least two cTnI examinations within the first 24 hours in the intensive care unit (ICU) after surgery were included in this study. There were no other prespecified exclusion criteria. Comprehensive clinical data (demographics, disease history, baseline echocardiographic data, and laboratory test results) were collected for each subject. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional ethics board of Beijing Anzhen Hospital (No. ks2019018). All participants provided informed consent before taking part.
Determination of cTnI and its longitudinal change
Blood samples were taken from patients by direct venipuncture when they returned to the ICU for determination of the cTnI level. Every patient had at least had two serum cTnI results at different time points. The first postoperative blood sample for testing cTnI (named cTnI-1) was drawn immediately after patients arrived at the surgical ICU (less than 1 hour after CABG), and the second blood sample (named cTnI-2) was drawn 12 to 18 hours after the procedure. The longitudinal change of cTnI within the first 24 hours was calculated as follows: cTnI-1 concentration − cTnI-2 concentration. The normal range for serum cTnI in our laboratory was 0.01 to 0.023 µg/L.
Outcomes
Outcome data were acquired by in-hospital record review, clinic visit, and telephone interview. Adverse events were independently confirmed by two authors in order to reduce bias. The primary endpoint of the study was the rate of a composite of 30-day all-cause mortality, stroke, myocardial infarction (MI), heart failure, and ventricular fibrillation after surgery. Outcome was defined in accordance with the Guidelines on myocardial revascularization (1).
Statistical analysis
Continuous data that were abnormally distributed are expressed as median [interquartile range (IQR)], and categorical data are presented as number (percentage). Change in cTnI was modelled as both categorical and continuous variables, and it is expressed as a categorical variable using quartiles and trends. Change expressed in trends was divided into four groups according to cTnI concentrations at the first and second sampling times relative to the median of each time: high to high, high cTnI-1 and high cTnI-2 concentrations; high to low, high cTnI-1 and low cTnI-2 concentrations; low to high, low cTnI-1 and high cTnI-2 concentrations; and low to low, low cTnI-1 and low cTnI-2 concentrations. Differences of baseline characteristics were compared using the Mann-Whitney U test or Kruskal-Wallis H test for continuous variables and the Pearson χ2 test or Fisher exact test for categorical variables. To examine the effect of the cTnI level at different time points and on clinical outcomes, proportional Cox hazard models were used to calculate the hazard ratio (HR). To analyze cTnI-1, cTnI-2, and absolute change, the models were adjusted for hypertension, left ventricular end-diastolic diameter (LVEDD), and left ventricular ejection fraction (LVEF), which were statistically different between the no event and event groups. Regarding longitudinal change, we adjusted LVEF for quartiles and sex, hemoglobin level, and platelet counts for trends, respectively. The receiver operating characteristic curve and area under the curve (AUC) were used to assess the ability of cTnI concentrations at different time points and their longitudinal change to predict 30-day adverse events. A two-sided P value <0.05 indicated a significant difference. All analyses were performed using Stata version 15.2 for Windows (StataCorp, College Station, TX, USA).
Results
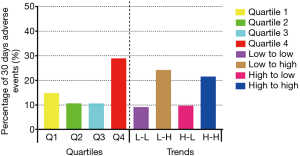
A total of 633 participants who underwent only off-pump CABG procedure (median age 63 years, range, 35–87 years, 74% men) with available early postoperative cTnI data were included in our study. The median EUROSCORE II was 1.01% (IQR 0.71–1.775%). The median cTnI concentrations were markedly elevated at first and second sampling times (0.140 µg/L, IQR 0.067–0.260 µg/L and 0.130 µg/L, IQR 0.063–0.340 µg/L, respectively). cTnI levels changed within 24 hours after the procedure. Table 1 shows the baseline characteristics of the study participants by two division methods: longitudinal change of cTnI expressed in quartiles and trends. A significant difference was observed in baseline LVEF across the four quartiles (P=0.031). When examining two cTnI levels relative to the median of each sampling time, we found that 15.2% of the patients changed from low to high concentrations, and 13.3% of the patients changed from high to low cTnI concentrations. By contrast, about 33.6% of the patients had persistently low cTnI concentrations, and 37.9% had persistently high cTnI levels. There were statistically significant differences in the proportion of men and preoperative hemoglobin and platelet levels among the four groups (P<0.05, all). In total, 101 patients (16.0%) experienced adverse events within 30 days postoperatively, comprising seven deaths, 13 patients with MI, 70 with heart failure, eight with ventricular fibrillation, and 13 with stroke (several patients sustained two or more complications).
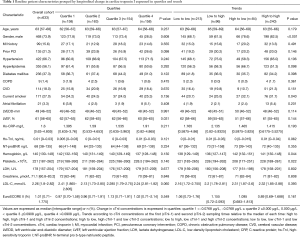
Full table
Participants who developed complications had larger LVEDD and lower LVEF, and they were more likely to have hypertension (P<0.05, all). Overall, 484 patients (76.5%) received three or more grafts for CABG.
Higher cTnI levels at the first and second sampling times after CABG were observed in the event group than in the no event group (P=0.002 and <0.001, respectively). Absolute change in cTnI levels which were defined as absolute value of first time cTnI concentration minus second time cTnI concentration was higher in the event group than in the no event group (P<0.001, Figure 1 and Table 2).
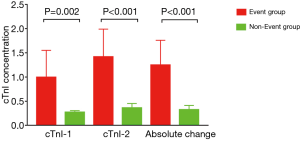
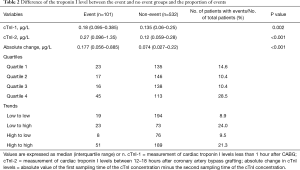
Full table
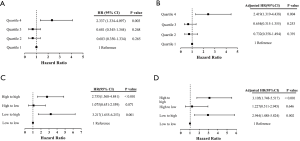
Both continuous cTnI-1 and dichotomized cTnI-1 levels at the cutoff of 0.065 µg/L, which was identified by the maximum Youden index value, were significantly associated with 30-day adverse events (adjusted HR for the continuous cTnI-1 level 1.598, 95% CI, 1.158–2.204; adjusted HR for the dichotomous cTnI-1 level 3.275, 95% CI, 1.613–6.651; Table 3). Significant associations were also observed between cTnI-2 and 30-day adverse events (adjusted HR for the continuous cTnI-2 level 1.499, 95% CI, 1.228–1.831; adjusted HR for the dichotomous cTnI-2 level 3.541, 95% CI, 2.177–5.759). Absolute change in cTnI was associated with a 38% increase in the risk of complications (adjusted HR for continuous absolute change 1.381, 95% CI, 1.138–1.674).
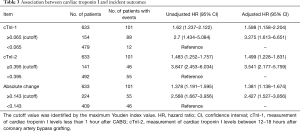
Full table
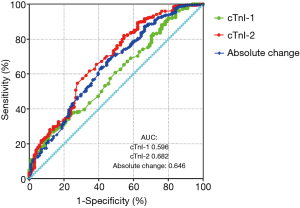
When longitudinal change in cTnI concentrations was ≥0.143 µg/L regardless of whether the trend was up or down, patients experienced a 142% increase in the risk of adverse events (adjusted HR for the dichotomous absolute change 2.427, 95% CI, 1.527–3.856, Table 3). The ability of cTnI-2 (AUC: 0.682) for predicting 30-day adverse events after CABG was relatively higher than the abilities of cTnI-1 and absolute change (AUC: 0.596 and 0.646, respectively; Figure 2).
Longitudinal change in cTnI within 24 hours after CABG was expressed in quartiles and trends. When presented in quartiles, we found that patients in quartile 1 (14.6%) and quartile 4 (28.5%) had a higher proportion of incident adverse events than those in quartile 2 (10.4%) and quartile 3 (10.4%). When expressed in trends, we found higher incidences of adverse events in the low to high (24.0%) and high to high (21.3%) groups than in the low to low (8.9%) and high to low (9.5%) groups (Table 2 and Figure 3). Patients in quartile 4 experienced a 2.34 times higher risk of adverse events than those in quartile 1 (HR 2.337, 95% CI, 1.334–4.097, P=0.003).
Further, this significant association remained even after adjustment for baseline LVEF (HR 2.419, 95% CI, 1.319–4.438, P=0.004; Figure 4). Patients with progression from low levels at the earlier time to high levels at the later time (low to high) showed a 2.94-fold higher incidence of 30-days adverse events after CABG than those with low to low levels (adjusted HR 2.944, 95% CI, 1.488–5.824, P=0.002). Moreover, patients whose cTnI concentrations changed from high to low had an adjusted HR of 1.227 (95% CI, 0.511–2.945, P=0.646). Those with persistently high cTnI levels had a 2.76 times higher risk of postoperative complications than those without (HR 2.755, 95% CI, 1.568–4.841, P<0.001); moreover, the association became more significant after adjusting for male sex and preoperative hemoglobin and platelet levels (adjusted HR 3.105, 95% CI, 1.748–5.517, P<0.001; Figure 4).
Discussion
The main findings of our study are as follows. First, we found that the cTnI concentration at a fixed time point within 24 hours after off-pump CABG is significantly associated with 30-day postoperative composite adverse events of all-cause mortality, heart failure, MI, stroke, and ventricular fibrillation. The significant association between cTnI levels within 1 hour after surgery and adverse events can provide a potential reference for more aggressive treatment to reduce the subsequent risk of complications. Second, we demonstrated that longitudinal change in the cTnI concentration within 24 hours postoperatively is independently associated with the development of 30-day composite adverse events. Specifically, patients with persistently high cTnI concentrations and those with progression from low to high cTnI concentrations had a more than three times increased risk of adverse events than those who had persistently low cTnI concentrations. Third, cTnI levels at a fixed time point and absolute change within 24 hours after CABG have some value for predicting adverse events (Figure 5).

CABG is among the most frequently performed cardiac surgical procedures (10,11), giving public concern to factors that adversely affect the outcomes of this procedure. The perioperative cTnI concentration is suggested to be one of the most sensitive and accurate myocardial biomarkers for identifying MI and predicting clinical outcomes following cardiovascular surgery (1,12,13); it even be used to discern between patients with graft-related MI, those with non-graft-related MI, and those without perioperative MI after CABG (14). A previous study reported that preoperative troponin measurement ahead of emergency CABG was an independent and powerful determinant of all-cause in-hospital mortality and major adverse cardiac events in acute ST-elevation MI and non-ST-elevation MI (15). However, other investigations suggested that there was no association between preoperative troponin and 30-day major adverse events in an elective CABG population or mixed population that included elective and emergency surgical patients (5,16), and even in the setting of general cardiac surgery, the preoperative troponin level was not associated with postoperative long-term mortality (17). Therefore, measurement of early postoperative troponin levels becomes necessary and meaningful, and it is not surprising that troponin levels are commonly elevated after CABG, as this elevation may result from insufficient myocardial protection during surgery, air embolism, graft occlusion, or other reasons (3,18-20).
Although the troponin concentration after CABG is affected by many factors (8), for instance, preoperative MI, kidney function, and LVEF, previous studies reported the promising ability of early troponin levels to predict clinical outcomes following the procedure. A large-scale prospective longitudinal study demonstrated that troponin was superior to electrocardiogram findings and creatinine kinase-MB with regard to predicting 5-year mortality after CABG (21); furthermore, the predictive value of troponin for 5-year mortality was validated in a separately collected cohort of 1,031 individuals who underwent CABG. In addition, another high-quality meta-analysis which included 18,908 patients (3), suggested that elevation of troponin levels within the first 24 hours after CABG was independently associated with increased mid- and long-term incidences of mortality. Nevertheless, the reality, which is easy to neglect, is that many adverse events occur immediately after CABG and urgent therapeutic measures are needed to improve the prognosis of these patients. Because the effective treatment time window narrows quickly, it is necessary to advance the time of measuring postoperative troponin levels. Gahl and colleague demonstrated that troponin levels measured between 6 and 12 hours after CABG can be used to identify individuals at an increased risk of major adverse events (7). Very few studies focused on the prognostic use of an early assessment of troponin within 6 hours after CABG to identify patients at an increased risk of complications. Our study demonstrated that a cTnI concentration at less than 1 hour after surgery was associated with composite adverse events, and this finding could potentially alert doctors to perform early therapeutic intervention to reduce patients’ risk. Serial measurement of troponin is commonly used in patients with persistent myocardial injury, which is suspected to lead to poor prognosis. Our study quantitively showed that longitudinal change between cTnI levels from two time points within first 24 hours after CABG was significantly associated with poor clinical outcomes, and that patients with persistently high cTnI levels and those with progression from low to high cTnI levels experienced a more than three-fold increased hazard of adverse events than patients who had persistently low cTnI concentrations.
Limitations
First, the single-center study design may have caused bias in the measurement of cTnI, and postoperative cTnI levels were partly affected by surgeons’ experience, the procedure duration, and other operative factors. Second, the study enrolled a relatively small number of patients who received off-pump CABG. Therefore, it would be valuable to confirm the association and replicate predictive capacity in an independently large cohort.
Conclusions
There was a significant association between early cTnI concentrations after CABG and adverse events even less than 1 hour after CABG. Longitudinal change in the cTnI level within 24 hours after CAGB is associated with the development of adverse events, and such data will be useful in identifying patients at an increased risk.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at http://dx.doi.org/10.21037/jtd-20-1691
Data Sharing Statement: Available at http://dx.doi.org/10.21037/jtd-20-1691
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd-20-1691). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional ethics board of Beijing Anzhen Hospital (No. ks2019018). All participants provided informed consent before taking part.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Neumann FJ, Sousa-Uva M, Ahlsson A, et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J 2019;40:87-165. [Crossref] [PubMed]
- Thielmann M, Massoudy P, Jaeger BR, et al. Emergency re-revascularization with percutaneous coronary intervention, reoperation, or conservative treatment in patients with acute perioperative graft failure following coronary artery bypass surgery. Eur J Cardiothorac Surg 2006;30:117-25. [Crossref] [PubMed]
- Domanski MJ, Mahaffey K, Hasselblad V, et al. Association of myocardial enzyme elevation and survival following coronary artery bypass graft surgery. JAMA 2011;305:585-91. [Crossref] [PubMed]
- El Messaoudi S, Wouters CW, van Swieten HA, et al. Effect of dipyridamole on myocardial reperfusion injury: A double-blind randomized controlled trial in patients undergoing elective coronary artery bypass surgery. Clin Pharmacol Ther 2016;99:381-9. [Crossref] [PubMed]
- Machado MN, Rodrigues FB, Grigolo IH, et al. Early Prognostic Value of High-Sensitivity Troponin T after Coronary Artery Bypass Grafting. Thorac Cardiovasc Surg 2019;67:467-74. [Crossref] [PubMed]
- Holmvang L, Jurlander B, Rasmussen C, et al. Use of biochemical markers of infarction for diagnosing perioperative myocardial infarction and early graft occlusion after coronary artery bypass surgery. Chest 2002;121:103-11. [Crossref] [PubMed]
- Gahl B, Gober V, Odutayo A, et al. Prognostic Value of Early Postoperative Troponin T in Patients Undergoing Coronary Artery Bypass Grafting. J Am Heart Assoc 2018;7:e007743. [Crossref] [PubMed]
- Koppen E, Madsen E, Greiff G, et al. Perioperative Factors Associated With Changes in Troponin T During Coronary Artery Bypass Grafting. J Cardiothorac Vasc Anesth 2019;33:3309-19. [Crossref] [PubMed]
- Fellahi JL, Gue X, Richomme X, et al. Short- and long-term prognostic value of postoperative cardiac troponin I concentration in patients undergoing coronary artery bypass grafting. Anesthesiology 2003;99:270-4. [Crossref] [PubMed]
- Alexander JH, Smith PK. Coronary-Artery Bypass Grafting. N Engl J Med 2016;374:1954-64. [Crossref] [PubMed]
- Lloyd-Jones D, Adams R, Carnethon M, et al. American Heart Association Statistics C and Stroke Statistics S. Heart disease and stroke statistics--2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 2009;119:480-6. [Crossref] [PubMed]
- Kim LJ, Martinez EA, Faraday N, et al. Cardiac troponin I predicts short-term mortality in vascular surgery patients. Circulation 2002;106:2366-71. [Crossref] [PubMed]
- Greenson N, Macoviak J, Krishnaswamy P, et al. Usefulness of cardiac troponin I in patients undergoing open heart surgery. Am Heart J 2001;141:447-55. [Crossref] [PubMed]
- Thielmann M, Massoudy P, Schmermund A, et al. Diagnostic discrimination between graft-related and non-graft-related perioperative myocardial infarction with cardiac troponin I after coronary artery bypass surgery. Eur Heart J 2005;26:2440-7. [Crossref] [PubMed]
- Thielmann M, Massoudy P, Neuhauser M, et al. Prognostic value of preoperative cardiac troponin I in patients undergoing emergency coronary artery bypass surgery with non-ST-elevation or ST-elevation acute coronary syndromes. Circulation 2006;114:I448-53. [Crossref] [PubMed]
- Petäjä L, Rosjo H, Mildh L, et al. Predictive value of high-sensitivity troponin T in addition to EuroSCORE II in cardiac surgery. Interact Cardiovasc Thorac Surg 2016;23:133-41. [Crossref] [PubMed]
- Lehrke S, Steen H, Sievers HH, et al. Cardiac troponin T for prediction of short- and long-term morbidity and mortality after elective open heart surgery. Clin Chem 2004;50:1560-7. [Crossref] [PubMed]
- Mohammed AA, Agnihotri AK, van Kimmenade RR, et al. Prospective, comprehensive assessment of cardiac troponin T testing after coronary artery bypass graft surgery. Circulation 2009;120:843-50. [Crossref] [PubMed]
- White HD. Pathobiology of troponin elevations: do elevations occur with myocardial ischemia as well as necrosis? J Am Coll Cardiol 2011;57:2406-8. [Crossref] [PubMed]
- Thygesen K, Alpert JS, Jaffe AS, et al. Fourth Universal Definition of Myocardial Infarction (2018). J Am Coll Cardiol 2018;72:2231-64. [Crossref] [PubMed]
- Muehlschlegel JD, Perry TE, Liu KY, et al. Troponin is superior to electrocardiogram and creatinine kinase MB for predicting clinically significant myocardial injury after coronary artery bypass grafting. Eur Heart J 2009;30:1574-83. [Crossref] [PubMed]


