
Central location and risk of imaging occult mediastinal lymph node involvement in cN0T2-4 non-small cell lung cancer
Introduction
Appropriate pre-operative staging is a cornerstone in the treatment of non-small cell lung cancer (NSCLC). Identifying patients with imaging occult mediastinal nodal involvement prior to surgery is important to prevent futile thoracoscopies. Factors which increase the risk of imaging occult mediastinal disease have been identified and their presence is used to decide on the need for preoperative invasive mediastinal staging by endoscopic needle techniques or mediastinoscopy. However, there are discrepancies between different guidelines on the characteristics which should lead to invasive mediastinal staging. The European Society of Thoracic Surgeons (ESTS) (1) and National Comprehensive Cancer Network (NCCN) (2) recommend invasive mediastinal staging in all tumors larger than 3 cm while the American College of Chest Physicians (ACCP) (3) does not consider tumor size in their recommendations. When examining the evidence cited by the ESTS and NCCN guidelines, only two studies demonstrating a low negative predictive value of integrated positron emission tomography (PET-CT) to detect occult mediastinal disease in this population are cited (4,5). Another characteristic cited by all three above mentioned guidelines is central tumor location. Although all guidelines cite central tumor location as an indication for invasive mediastinal staging, the definition of a central tumor remained elusive until recently. This was well highlighted by a survey published by Casal et al. (6) demonstrating large discrepancies between physicians in what was considered a central tumor. Since this survey, Shin et al. (7) explored different definitions of central tumor location in a large cohort of radiologically N0 NSCLC and found only one definition which was significantly associated with the presence of occult mediastinal disease: a lesion located within the inner one-third of the hemithorax delimited by concentric lines arising from the midline. In another study, Casal et al. (8) also identified two definitions associated with upstaging to any N in T1N0M0 patients but the association did not remain when focusing on N2/3 disease. Larger tumors have a greater chance of having a central portion due to their size, hence of being considered central, this may explain the relationship between tumor size and imaging occult mediastinal disease. Tumor size could also be a predictor of occult mediastinal disease, independently from tumor location.
Given this background, we conducted a retrospective study to compare the prevalence of occult mediastinal disease in central and peripheral radiological N0 NSCLC larger than 3 cm. We present the following article in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting checklist (available at http://dx.doi.org/10.21037/jtd-20-1565).
Methods
Study population
Using the prospectively maintained database of the lung cancer investigation clinic of the Institut Universitaire de Cardiologie et de Pneumologie de Québec (IUCPQ), we retrospectively identified all patients with radiological N0 NSCLC which were investigated in our institution from 01/01/2014 to 31/12/2018. The study was approved by local Research Ethics Board (CRIUCPQ, 2019-3070), and informed consent was taken from all the patients. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
All patients with resectable cT2-4N0 NSCLC who underwent surgical lymph node (LN) dissection or invasive mediastinal staging demonstrating N2/3 disease were included. All surgical resections were performed by video-assisted thoracic surgery (VATS) or thoracotomy with systematic mediastinal nodal dissection.
Patients who did not undergo confirmatory surgical lymph nodes dissection after a negative invasive staging procedure, with synchronous lung tumors, previous history of lung cancer and final pathology of small cell lung cancer, carcinoid tumor or pulmonary metastasis from another organ were excluded. Patients who did not undergo CT and PET-CT or for whom radiology reports of CT and PET-CT were not available were excluded.
Imaging
A lymph node was considered abnormal on imaging if its short axis was greater or equal to 10mm on computed tomography (CT) scan or its maximum standardized uptake value (SUV) was greater or equal to 2.5 on integrated positron emission tomography (PET-CT).
Tumor location was determined from axial, sagittal and coronal images if a CT was available. If CT images were not available, axial images from PET-CT were used. Location was defined based on the most central portion of the tumor. Central tumor was defined as located in the inner one-third of the hemithorax adopted by drawing concentric lines from the midline, based on a recent study (7).
All images were blindly reviewed by MF and JG. If location reported by MF and JG differed, a senior radiologist reviewed images and made the final decision. All tumor sizes were measured based on their long axis on CT. Tumors were classified according to 8th TNM classification (9). For T3 and T4 NSCLC based on the presence of multiple lesions in the same lung, we measured the largest lesion to determine tumor size.
Objectives
Our primary objective was to compare the prevalence of occult mediastinal disease in central and peripheral radiological N0 NSCLC larger than 3 cm. Our secondary objectives were to assess the impact of tumor size and location on the prevalence of any occult nodal disease (pN1-3) and the negative predictive value of PET-CT for pN1-3 and pN2/N3 disease according to tumor location.
Statistics
Data was compared using the Chi-square or Fisher tests for categorical variables, and the t-test or Mann-Whitney test for continuous variables depending on the Gaussian distribution of the variables. OR and 95% confidence intervals (CIs) were calculated from binary logistic regression models for pN1-3 upstaging (versus pN0) and pN2/N3 upstaging (versus pN0/N1). A multivariate logistic regression analysis was used to adjust for potential confounding factors in the association between central tumour and occult N2 disease. All statistical results were 2-sided and a P value <0.05 was considered significant. All analysis were performed with the Prism software (GraphPad Software Inc., California, United States) and SAS software (version 9.4 of the SAS System for Windows).
Results
Patient and tumors characteristics
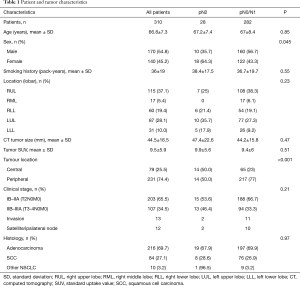
Three hundred and ten cT2-4N0 NSCLC staged with a CT and PET-CT who met inclusion criteria were found in our local lung cancer investigation clinic database for the January 2014 to December 2018 period. Patient and tumor characteristics are listed in Table 1. Mean age was 66.8 years and 54.8% of patients were females. Most tumors were T2 (cIB–IIA; n=203, 65.5%). Within T3/T4 subgroup (cIIB–IIIA), 13 (12.1%) were classified as T3/T4 invasion and 12 (11.2) as T3/T4 ipsilateral nodule. CT scan images were available for axial, coronal and sagittal image review to determine tumor location in 251 patients (81.3%). Seventy-nine (25.5%) tumors were classified as central and 231 (74.5%) were peripheral.
Full table
Surgical characteristics
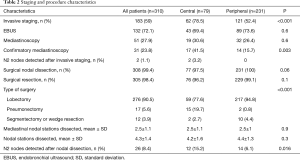
One hundred and eighty-three patients (59.0%) underwent invasive mediastinal staging prior to surgery, mainly by endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) (72.1%) (Table 2). Centrally located tumors were associated with an increased rate of invasive mediastinal staging (78.5% vs. 52.4%), and confirmatory mediastinoscopy after a negative EBUS staging (43.7% vs. 12.5%). Two occult N2 metastases were found after EBUS staging and these two patients were not operated. All other patients underwent surgical mediastinal nodal dissections (Table 2). The mean number of dissected nodal stations was 4.3±1.4, including 2.5±1.1 mediastinal nodal stations. No differences in the number of nodal stations assessed during the surgery were reported between central and peripheral tumors.
Full table
Risk factors for imaging occult mediastinal disease (N2-3)
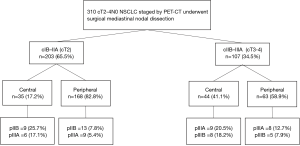
The prevalence of imaging occult mediastinal disease was 9.9% (n=28). Age, lesion SUV, histology and tumor size were not associated with a higher prevalence of pN2-3 disease (Table 1). Female sex was associated with a lower prevalence of pN2-3 disease [10/170 (5.9%) vs. 18/140 (12.9%), P=0.045] while central tumor location was associated with a higher prevalence of pN2 disease [14/79 (17.7%) vs. 14/231 (6.1%), P<0.001]. The risk of nodal upstaging according to the clinical stage and the location of the tumor is detailed in Figure 1. Amongst cIB-IIA tumors, 17.1% of central and 5.4% of peripheral tumors were upstaged to stage pIIIA while amongst cIIB-IIIA tumors, 18.2% of central and 7.9% of peripheral tumors were upstaged to stage pIIIB.
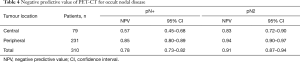
In a multivariate analysis, tumor central location remained the only factor statistically associated with imaging occult mediastinal disease (adjusted OR 3.23, 95% CI: 1.45-7.18, P=0.001) (Table 3). NPV of PET-CT for occult mediastinal disease was 0.83 (95% CI: 0.72–0.90) in central and 0.94 (95% CI: 0.90–0.97) in peripheral tumor (Table 4).

Full table
Full table
Risk factors for imaging any occult nodal disease (N1-3)
The prevalence of any imaging occult nodal disease was 22.3% (n=69). Prevalence was 43% (n=34) in central tumors and 15.2% (n=35) in peripheral tumors.
In a multivariate analysis, tumor central location was statistically associated with imaging occult nodal disease (adjusted OR 5.0; 95% CI: 2.59–9.62, P<0.001) (Table 3). NPV of PET-CT for any occult nodal disease was 0.57 (95% CI: 0.45–0.68) in central and 0.80 (95% CI: 0.73–0.82) in peripheral tumor (Table 4).
Discussion
In this retrospective study, the overall prevalence of occult mediastinal disease in cT2-4N0M0 NSCLC was 9%, with a significantly lower prevalence in peripheral vs. central tumors (6.1 vs. 17.7%). Tumor size was not significantly associated with the prevalence of occult mediastinal disease in this population. This questions the need to have “tumor larger than 3 cm” amongst indications for invasive mediastinal staging as if larger tumors are peripheral, their prevalence of occult mediastinal disease remains relatively low irrespective of their size.
Guidelines provided by the ESTS and the NCCN include tumor size above 3 cm as an indication for invasive mediastinal staging. This recommendation is based on the results of two studies. The first is a meta-analysis which reported a 89% NPV for PET-CT to detect any nodal upstaging in NSCLC larger than 3 cm while the NPV was 94% in NSCLC smaller than 3 cm (4). Tumor location was not considered in this meta-analysis. One could speculate that larger tumors are more likely to be central due to their size which could have driven the findings. The second is a smaller single center study limited to 153 peripheral tumors which found a NPV for PET-CT to detect mediastinal disease of 85% in peripheral NSCLC larger than 3 cm while the NPV was 92% in peripheral NSCLC smaller than 3 cm (5). Tumor centrality was defined by the absence of contact with the intrapulmonary main bronchi, pulmonary artery, pulmonary veins, or the origin of the first segmental branches. This definition never proved to be associated with occult mediastinal disease in subsequent studies and may have misclassified certain tumors as peripheral thus decreasing the NPV of PET in peripheral tumors.
Since the publication of these guidelines, studies attempting to define tumor centrality have been published to reconciliate previous studies which yielded variable conclusions as to the relationship between central tumor location and occult mediastinal disease. Casal et al. focused on cT1N0M0 patients and failed to identify a definition of central tumor associated with an increased prevalence of occult N2/N3 disease despite a robust 607 patients study exploring 8 different definitions (8). Decaluwé et al. did not limit themselves to T1 lesions but again were not able to find a definition of central tumor associated with an increased prevalence of occult N2/N3 disease (10). Both studies did find an association between tumor centrality and upstaging to any N. In a third study, Shin et al. included tumors irrespective of size and found only one definition which predicted occult N2 disease: a lesion located within the inner one-third of the hemithorax delimited by concentric lines arising from the midline. They hypothesize that this definition, which they were the only group to study, may have been associated with occult mediastinal disease due to skip metastasis to the mediastinum from apical tumors which are not considered central according to the more commonly used definitions which are based on concentric circles arising from the hilums or contact with central structures. As this study focused on defining tumor centrality, the impact of other factors, such as tumor size, on the prevalence of occult disease mediastinal was not explored after adjustment for tumor location. Univariate analysis did demonstrate a significant relationship between tumor size and occult mediastinal disease. The fact that we did not find this relationship in our cohort may be explained by the exclusion of smaller tumors. Yang et al. (11) previously demonstrated that the relationship between tumor size and occult mediastinal disease dissipates for further increase in tumor size above 3 cm. Likewise, tumor location was determined by physicians. However, all images were blindly and independently reviewed by two pulmonologists, and in case of divergence by a senior radiologist. Moreover, most of tumor location were defined from a CT scan using axial, coronal and sagittal views and not only from axial images, in order to obtain the most relevant classification.
The prevalence of occult mediastinal disease in peripheral cT2N0 remains low in our study (5.4%) but others, such as Gao et al. (12), obtained higher prevalences in their population. However, Gao et al. (12) did not focus on this subgroup and included only 48 solid cT2N0 but obtained a prevalence of 18% in peripheral cT2N0 cancer. The specific prevalence of occult mediastinal disease for the peripheral cT2N0 subgroup is not available for most studies, but Gómez-Caro et al. (5) and Decaluwé et al. reported respectively only 9.2% and 11.7% for the cT2N0 subgroup including peripheral tumors.
We elected to limit our study to cN0 patients as cN1 patients already have a well demonstrated indication for invasive mediastinal staging with a 25% prevalence of occult mediastinal disease regardless the location of the tumor (13,14). We also excluded cT1N0M0 tumors as our team and Casal et al. (8) have previously demonstrated that despite being central, cT1N0M0 tumors remain at low prevalence of occult mediastinal disease.
Preoperative knowledge of N1 disease may modify management if radiation therapy or a sublobar resection are the planned treatment and this should be considered when staging a patient if these treatments are planned. CT and PET CT were associated with an 80% negative predictive value for detecting N1-3 disease in peripheral cT2-4N0 NSCLC hence further staging is necessary in this population prior to radiation therapy or sublobar resection. If the patient is a lobar resection candidate, occult N1 disease discovered at surgery will modify postoperative but not intraoperative or preoperative management. Because of the heterogeneity of patients with locally advanced stage III NSCLC, making an optimal decision for management of these patients is complex. Surgery has long been considered as the optimal treatment for resectable disease. It should be emphasized that multi-modality therapy combining chemotherapy and radiation therapies in addition to surgery (either as neo-ajuvant or adjuvant) improve outcomes compared to surgery alone (15). For unresectable stage III disease, concurrent chemoradiation is the preferred treatment and consolidation with durvalumab reported good outcomes in term of progression free survival (16).
Conclusions
This retrospective study suggests that invasive mediastinal staging is required in central cT2-4N0 NSCLC but can be questioned in peripheral one, especially in cT2N) subgroup if the patient is a candidate for lobar resection. Further larger prospective studies to validate this finding are necessary.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/jtd-20-1565
Data Sharing Statement: Available at http://dx.doi.org/10.21037/jtd-20-1565
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd-20-1565). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by local Research Ethics Board (CRIUCPQ, 2019-3070), and informed consent was taken from all the patients. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- De Leyn P, Dooms C, Kuzdzal J, et al. Revised ESTS guidelines for preoperative mediastinal lymph node staging for non-small-cell lung cancer. Eur J Cardiothorac Surg 2014;45:787-98. [Crossref] [PubMed]
- National Comprehensive Cancer Network. Non-Small Cell Lung Cancer (Version 5.2018). Available online: https://www.nccn.org/professionals/physician_gls/PDF/nscl.pdf.
- Silvestri GA, Gonzalez AV, Jantz MA, et al. Methods for staging non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e211S-50S.
- Wang J, Welch K, Wang L, et al. Negative predictive value of positron emission tomography and computed tomography for stage T1-2N0 non-small-cell lung cancer: a meta-analysis. Clin Lung Cancer 2012;13:81-9. [Crossref] [PubMed]
- Gómez-Caro A, Boada M, Cabañas M, et al. False-negative rate after positron emission tomography/computer tomography scan for mediastinal staging in cI stage non-small-cell lung cancer. Eur J Cardiothorac Surg 2012;42:93-100; discussion 100. [Crossref] [PubMed]
- Casal RF, Vial MR, Miller R, et al. What Exactly Is a Centrally Located Lung Tumor? Results of an Online Survey. Ann Am Thorac Soc 2017;14:118-23. [Crossref] [PubMed]
- Shin SH, Jeong DY, Lee KS, et al. Which definition of a central tumour is more predictive of occult mediastinal metastasis in non-small cell lung cancer patients with radiological N0 disease? Eur Respir J 2019;53:1801508. [Crossref] [PubMed]
- Casal RF, Sepesi B, Sagar AES, et al. Centrally located lung cancer and risk of occult nodal disease: an objective evaluation of multiple definitions of tumour centrality with dedicated imaging software. Eur Respir J 2019;53:1802220. [Crossref] [PubMed]
- Detterbeck FC, Boffa DJ, Kim AW, et al. The Eighth Edition Lung Cancer Stage Classification. Chest 2017;151:193-203.
- Decaluwé H, Moons J, Fieuws S, et al. Is central lung tumour location really predictive for occult mediastinal nodal disease in (suspected) non-small-cell lung cancer staged cN0 on 18F-fluorodeoxyglucose positron emission tomography-computed tomography? Eur J Cardiothorac Surg 2018;54:134-40. [Crossref] [PubMed]
- Yang F, Chen H, Xiang J, et al. Relationship between tumor size and disease stage in non-small cell lung cancer. BMC Cancer 2010;10:474. [Crossref] [PubMed]
- Gao SJ, Kim AW, Puchalski JT, et al. Indications for invasive mediastinal staging in patients with early non-small cell lung cancer staged with PET-CT. Lung Cancer 2017;109:36-41. [Crossref] [PubMed]
- Dooms C, Tournoy KG, Schuurbiers O, et al. Endosonography for mediastinal nodal staging of clinical N1 non-small cell lung cancer: a prospective multicenter study. Chest 2015;147:209-15. [Crossref] [PubMed]
- Decaluwé H, Dooms C, D’Journo XB, et al. Mediastinal staging by videomediastinoscopy in clinical N1 non-small cell lung cancer: a prospective multicentre study. Eur Respir J 2017;50:1701493. [Crossref] [PubMed]
- Yoon SM, Shaikh T, Hallman M. Therapeutic management options for stage III non-small cell lung cancer. World J Clin Oncol 2017;8:1-20. [Crossref] [PubMed]
- Antonia SJ, Villegas A, Daniel D, et al. Durvalumab after Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. N Engl J Med 2017;377:1919-29. [Crossref] [PubMed]


