Low molecular weight heparin once versus twice for thromboprophylaxis following esophagectomy: a randomised, double-blind and placebo-controlled trial
Introduction
Esophageal cancer is one of the most common cancers and esophagectomy is regarded as the standard therapy for esophageal cancer. Venous thromboembolism (VTE), including deep vein thrombosis (DVT) and pulmonary embolism (PE), is a set of life-threatening complications associated with surgery (1-3). It was reported that incidence of VTE was 1.3% to 24% (4-10). The risk factors of VTE including elderly age, female sex, obesity, bed-rest, venous catheterization, radiotherapy and infections (11-15). Patients undergoing thoracic surgery, especial esophagectomy, are often elderly and reluctant to early ambulation due to postoperative fatigue and the pain from chest tubes (16). De Martino et al. (17) reported that the incidence of DVT, PE, and VTE within 1 month after esophagectomy for esophageal cancer is 6.1%, 2.4%, and 7.3%, respectively. Reciprocally, VTE increases the mortality of cancer patients (15).
Thromboprophylaxis has been accepted as a routine after surgery in patients with high risks of VTE and low molecular weight heparin (LMWH) has been recommended as the standard prophylactic anticoagulants according to the latest version of guideline by the American College of Chest Physicians (ACCP) (18). Although the guideline suggested initiating the daily use of LMWH in non-orthopedic surgery patients at 12 hours after surgery, the optimal dose and timing of LMWH for thromboprophylaxis in patients undergoing esophagectomy is still uncertain. Recently we reported a case that who experienced fatal PE after esophagectomy despite thromboprophylaxis with daily LMWH (19).
There are few randomized controlled trials on the different dose of LMWH for thromboprophylaxis in patients with esophageal. In the present study, we compared the safety and efficiency between LMWH once-daily (QD) and LMWH twice-daily (BID) for the prophylaxis of VTE in patients following esophagectomy.
Methods
Participants
The protocol was approved by the Ethics Committee of Zhongshan Hospital (No. 2010-186) and registered in ClinicalTrials.gov (NCT01267305). Patients meeting the following criteria were evaluated for eligibility: (I) 18-75 years; (II) conformed esophageal cancer; (III) elected for esophagectomy. Written informed consent was obtained from all patients.
Exclusion criteria included: (I) prothrombin time (PT) or activated partial thromboplastin time (APTT) >1.5 times the upper normal limit; (II) blood platelet count <50×1012/L; (III) anticoagulant or antiplatelet history before surgery; (IV) any history of hemorrhagic disease; (V) any history of intracranial, spinal or ophthalmologic operation; (VI) history of peptic ulcer; (VII) bleeding >400 mL in operation or >100 mL/h during the first 6 hours after the operation, or blood transfusion within 6 hours after the operation; (VIII) severe renal or liver dysfunction.
Trial design and interventions
This was a single center study conducted in Zhongshan Hospital in Shanghai, China, from August 2012 to July 2013. The study was designed as a randomized, double-blinded, placebo-controlled, parallel-group trial. Participants were randomly assigned to receive nadroparin calcium (Fraxiparine, GlaxoSmithKli, UK) 4,100 AxaIU and placebo once-daily respectively (group QD) or nadroparin calcium twice-daily (group BID), starting from 6 hours after esophagectomy (18). For allocation of the participants, a computer-generated list of random numbers was used. Blinding and equipoise were strictly maintained by emphasizing to intervention staff and participants, that the appearance and timing of administration of placebo in Group QD were the same as the second dose of nadroparin calcium in group BID. The treatment was discontinued on the 7th day after surgery or upon any of the following bleeding event: fatal bleeding, postoperative intracranial bleeding, reoperation for controlling bleeding, transfusion of 5U or more packed red blood cell (RBC) within a 48-hour period, and chest drainage >2 L within a 24-hour period.
Outcomes
The primary outcome with respect to efficacy of LMWH for thromboprophylaxis was the morbidity of VTE at 7th postoperative day. Vascular ultrasound was carried out daily by the same experienced operator to detect DVT in the lower limbs until the 7th day after surgery. Once PE was suspected, pulmonary CT angiography or pulmonary arteriography was performed to make a definite diagnosis.
The secondary outcome of the trial was the blood coagulation status of the enrolled patients which was assessed with a thromboelastogram (TEG) analyzer (TEG 5000 Hemostasis analyzer, Haemoscope Corporation, Niles, IL, USA) before and at 0/24/48/72 hours after operation (20). Specific parameters of TEG included: reaction time (R, min), the time elapsed from initiation of the test to the initial fibrin formation; coagulation time (K, min), the time from the beginning of a clot formation until the amplitude of TEG reaches 20 mm; alpha angle (α, degrees), the angle formed by the slope of a tangent line traced from the R to the K; maximum amplitude (MA, mm), measurement of maximal strength or stiffness of the developed clot.
For evaluation of safety of these two anti-coagulative strategies, the cumulative chest drainage, including the volume and RBC count, were examined at the 72 hours after the surgery. Any thromboembolic events or bleeding events were also recorded.
Statistical analysis
Statistical analysis was performed with SPSS version 21 (IBM Corporation, Armonk, NY, USA). Data are presented as mean ± standard deviation. Continuous variables were analyzed with ANOVA of repeated measure. Categorical variables were compared by the chi-square test or Fisher’s exact test. A P value of <0.05 (2-sided) was considered to be statistically significant.
Results
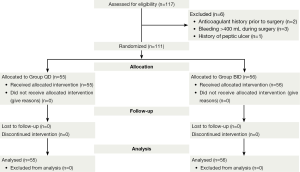
A total of 117 patients were enrolled in this study, and 111 eligible patients were randomly assigned to two arms: 55 patients in group QD and the remaining 56 patients in group BID (Figure 1). The demographics were comparable between the two arms (Table 1).

Full table
Four patients in the group QD and no patient in group BID were diagnosed DVT by lower limbs ultrasound (7.27% versus 0%, P=0.046). One symptomatic PE was observed in group D on the 5th day after the surgery which was conformed to pulmonary arteriography (Table 2). The incidence of VTE was lower in group BID when compared with group QD (9.09% versus 0%, P=0.032).
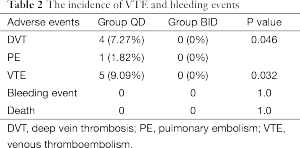
Full table
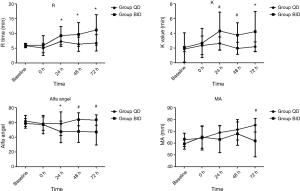
Prior to and instantly after the operation, all TEG measures, including the R time, K time, α-angle and MA values, were within the normal range and comparable between the two groups (Figure 2). Then TEG values were analyzed on each of the 3 postoperatively consecutive days in both groups. The R time of both groups prolonged after receiving anticoagulants, but the change was more remarkable in group BID during the first 3 postoperative days (P<0.05). In additional, group BID had longer K time and smaller α-angle (P<0.05). Compared with group QD, MA of group BID decreased significantly on the third postoperative day (P<0.01), but not on the first and the second postoperative days (Figure 2).
The cumulative chest drainage volume at 72 hours after the surgery were comparable between these two groups (1,001.39±424.58 versus 1,133.61±513.93 mL, P=0.406). RBC counts in chest drainage were also identical between the two groups [(2.56±1.98)×105 versus (2.71±4.67)×105, P=0.61]. No bleeding event that necessitates ending the trial was noticed. No patient died due to VTE or bleeding events during the trial (Table 2).
Discussion
This was the first study to investigate the effect of different dosage of LMWH on thromboprophylaxis after thoracic surgery. In our study, we found that BID LMWH decreased the morbidity of VTE after esophagectomy. To quantificationally monitor the efficacy and safety of the increased dose of LMWH, we used TEG to assess the profile of the coagulation. We observed that BID LMWH significantly crippled the coagulability of these patients during first postoperative 72 hours.
LMWH is derived from unfractionated heparin (UFH) by chemical or enzymatic depolymerization and its main effect is the inhibition of the coagulation factor Xa and thrombin. The half-life of LMWH ranges from 3 to 6 hours after subcutaneous injection (21). It was safe in prophylactic dosage and coagulation monitoring is not generally necessary. Several guidelines recommend using LMWH for thromboprophylaxis in high-risk patients undergoing thoracic surgery (18,22). However, the optimal dose of LMWH referring to thromboprophylaxis is still controversial. Whether increased dose of LMWH could further decrease the incident of perioperative VTE in patients undergoing thoracic surgery? In our study, we randomly assigned the enrolled patients to receive LMWH either QD or BID for thromboprophylaxis.
The anticoagulation effect of LMWH was tested by TEG, which could monitor global coagulation state more effectively than traditional measures such as PT and APTT (23). TEG has been increasingly used to monitor coagulation function after surgical procedures (24), and has been validated as a measure to monitor the dosage of LMWH (20,25-27). Several studies indicated that TEG is sensitive to hypercoagulation status associated with thrombo-embolic events (20,23,28). In our research, TEG was used to monitor the dose-dependent anticoagulation effect of LMWH mainly with prolonged R time.
Zmuda et al. (25) showed that LMWH could cause a dose-dependent inhibition of clotting. In our study, prior to and instantly after the surgery, all four TEG measures were within normal range and comparable between the two groups. Both dosage of LMWH made hypo-coagulative status with prolonged R time, K time and decreased α-angle. The changes, however, were more pronounced in the group BID. This result demonstrated that patients receiving BID LMWH had more adequate anticoagulation effect compared to those receiving LMWH just once. More direct measures showed that the incidence of VTE in group QD was comparable to the previous studies (8,17), while that of group BID was significant lower. These results suggest that BID LMWH was more efficacious in preventing VTE following esophagectomy. Udy et al. (29) found that 65.1% patients who had an expected ICU length of stay more than 24 hours manifested augmented renal clearance on at least one occasion during the first 7 study days. Augmented renal clearance might result in inadequate plasma concentration of pharmaceuticals which were excreted via kidney, such as LMWH. Considering the high risks of VTE in esophagectomy patients as we mentioned above and the probable augmented renal clearance of LMWH, daily LMWH in postoperative thromboprophylaxis in patients of normal renal function seemed to be not enough.
Numerous randomized clinical trials have shown the safety and efficiency of LMWH in prevention of VTE, so coagulation monitoring was not necessary except in those obese or renal insufficient patients (30-32). In the current study, the postoperatively cumulative chest drainage volume at 72 hours was comparable between group QD and group BID. Meanwhile, the RBC count in the chest drainage did not differed between the two groups which meant that BID LMWH for the prophylaxis of VTE did not increase the bleeding risk in comparison of QD LMWH. So we believe that twice LMWH in postoperative thromboprophylaxis in esophageal cancer patients undergoing esophagectomy was safe.
The limitation of this study included that it was a single center trial with limited number of subjects. One the other hand, the TEG exams were performed every morning, not at a particular time related to the administration of LMWH. So the anticoagulation effects of peak plasma level could be missed. So the results must be interpreted carefully, and further study based on larger population is required to confirm these findings.
In summary, the current study suggested that BID LMWH provided more potent efficacy and equal safety in the prophylaxis of VTE when compared to QD LMWH in patients undergoing selective esophagectomy.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Kakkar AK, Haas S, Wolf H, et al. Evaluation of perioperative fatal pulmonary embolism and death in cancer surgical patients: the MC-4 cancer substudy. Thromb Haemost 2005;94:867-71. [PubMed]
- Bergqvist D. Venous thromboembolism and cancer: prevention of VTE. Thromb Res 2001;102:V209-13. [PubMed]
- Kakkar AK. Prevention of venous thromboembolism in the cancer surgical patient. J Clin Oncol 2009;27:4881-4. [PubMed]
- Lee HM, Suk KS, Moon SH, et al. Deep vein thrombosis after major spinal surgery: incidence in an East Asian population. Spine (Phila Pa 1976) 2000;25:1827-30. [PubMed]
- Pookarnjanamorakot C, Sirisriro R, Eurvilaichit C, et al. The incidence of deep vein thrombosis and pulmonary embolism after total knee arthroplasty: the screening study by radionuclide venography. J Med Assoc Thai 2004;87:869-76. [PubMed]
- Nathan S, Aleem MA, Thiagarajan P, et al. The incidence of proximal deep vein thrombosis following total knee arthroplasty in an Asian population: a Doppler ultrasound study. J Orthop Surg (Hong Kong) 2003;11:184-9. [PubMed]
- Lapidus LJ, Ponzer S, Pettersson H, et al. Symptomatic venous thromboembolism and mortality in orthopaedic surgery - an observational study of 45 968 consecutive procedures. BMC Musculoskelet Disord 2013;14:177. [PubMed]
- Rollins KE, Peters CJ, Safranek PM, et al. Venous thromboembolism in oesophago-gastric carcinoma: incidence of symptomatic and asymptomatic events following chemotherapy and surgery. Eur J Surg Oncol 2011;37:1072-7. [PubMed]
- Kanchanabat B, Stapanavatr W, Manusirivithaya S, et al. The rate and mortality of postoperative venous thromboembolism of moderate risk surgery in Asian patients without thrombo-prophylaxis: systematic review with meta-analysis. World J Surg 2014;38:194-202. [PubMed]
- Jamal MH, Corcelles R, Shimizu H, et al. Thromboembolic events in bariatric surgery: a large multi-institutional referral center experience. Surg Endosc 2015;29:376-80. [PubMed]
- Khorana AA, Francis CW, Culakova E, et al. Risk factors for chemotherapy-associated venous thromboembolism in a prospective observational study. Cancer 2005;104:2822-9. [PubMed]
- Shah MA, Capanu M, Soff G, et al. Risk factors for developing a new venous thromboembolism in ambulatory patients with non-hematologic malignancies and impact on survival for gastroesophageal malignancies. J Thromb Haemost 2010;8:1702-9. [PubMed]
- Khorana AA, Connolly GC. Assessing risk of venous thromboembolism in the patient with cancer. J Clin Oncol 2009;27:4839-47. [PubMed]
- Bosch DJ, Van Dalfsen QA, Mul VE, et al. Increased risk of thromboembolism in esophageal cancer patients treated with neoadjuvant chemoradiotherapy. Am J Surg 2014;208:215-21. [PubMed]
- Trinh VQ, Karakiewicz PI, Sammon J, et al. Venous thromboembolism after major cancer surgery: temporal trends and patterns of care. JAMA Surg 2014;149:43-9. [PubMed]
- Ziomek S, Read RC, Tobler HG, et al. Thromboembolism in patients undergoing thoracotomy. Ann Thorac Surg 1993;56:223-6; discussion 227. [PubMed]
- De Martino RR, Goodney PP, Spangler EL, et al. Variation in thromboembolic complications among patients undergoing commonly performed cancer operations. J Vasc Surg 2012;55:1035-40. [PubMed]
- Guyatt GH, Akl EA, Crowther M, et al. Executive summary: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012;141:7S-47S.
- Zhong M, Tan L, Xue Z, et al. Extracorporeal membrane oxygenation as a bridge therapy for massive pulmonary embolism after esophagectomy. J Cardiothorac Vasc Anesth 2014;28:1030-2. [PubMed]
- Attaran S, Somov P, Awad WI. Randomised high- and low-dose heparin prophylaxis in patients undergoing thoracotomy for benign and malignant disease: effect on thrombo-elastography. Eur J Cardiothorac Surg 2010;37:1384-90. [PubMed]
- Garcia DA, Baglin TP, Weitz JI, et al. Parenteral anticoagulants: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012;141:e24S-43S.
- Venous thromboembolism. Reducing the risk of venous thromboembolism (deep vein thrombosis and pulmonary embolism) in patients undergoing surgery. NICE clinical guidelines 46; April 2007. Available online: http://www.venous-thromboembolism.org/reports/CG046NICEguideline.pdf
- Park MS, Martini WZ, Dubick MA, et al. Thromboelastography as a better indicator of hypercoagulable state after injury than prothrombin time or activated partial thromboplastin time. J Trauma 2009;67:266-75; discussion 275-6. [PubMed]
- Spiess BD, Gillies BS, Chandler W, et al. Changes in transfusion therapy and reexploration rate after institution of a blood management program in cardiac surgical patients. J Cardiothorac Vasc Anesth 1995;9:168-73. [PubMed]
- Zmuda K, Neofotistos D, Ts'ao CH. Effects of unfractionated heparin, low-molecular-weight heparin, and heparinoid on thromboelastographic assay of blood coagulation. Am J Clin Pathol 2000;113:725-31. [PubMed]
- Gonzalez E, Kashuk JL, Moore EE, et al. Differentiation of enzymatic from platelet hypercoagulability using the novel thrombelastography parameter delta (delta). J Surg Res 2010;163:96-101. [PubMed]
- Forfori F, Ferro B, Mancini B, et al. Role of thrombolestagrophy in monitoring perioperative coagulation status and effect of thromboprophylaxis in bariatric surgery. Obes Surg 2012;22:113-8. [PubMed]
- McCrath DJ, Cerboni E, Frumento RJ, et al. Thromboelastography maximum amplitude predicts postoperative thrombotic complications including myocardial infarction. Anesth Analg 2005;100:1576-83. [PubMed]
- Udy AA, Baptista JP, Lim NL, et al. Augmented renal clearance in the ICU: results of a multicenter observational study of renal function in critically ill patients with normal plasma creatinine concentrations*. Crit Care Med 2014;42:520-7. [PubMed]
- Abbate R, Gori AM, Farsi A, et al. Monitoring of low-molecular-weight heparins in cardiovascular disease. Am J Cardiol 1998;82:33L-36L. [PubMed]
- Francis CW, Pellegrini VD Jr, Totterman S, et al. Prevention of deep-vein thrombosis after total hip arthroplasty. Comparison of warfarin and dalteparin. J Bone Joint Surg Am 1997;79:1365-72. [PubMed]
- Samama MM, Poller L. Contemporary laboratory monitoring of low molecular weight heparins. Clin Lab Med 1995;15:119-23. [PubMed]