Anemia resolved by thoracoscopic resection of a mediastinal mass: a case report of unicentric Castleman’s disease
Introduction
Castleman’s disease (CD) is a rare form of lymph node hyperplasia, and most commonly presents as a solitary mediastinal mass with an indolent course (1,2). The pathophysiology of CD is still unknown (1,2). Making the correct diagnosis of CD preoperatively is difficult because unicentric CD rarely occurs in patients with anemia and easily mimics other mediastinal tumors, such as lymphoma, thymoma, and neurogenic tumors (3,4). We report here an unusual case of mediastinal unicentric CD with anemia that resolved after thoracoscopic resection.
Case report
A 25-year-old woman was referred with an abnormal chest X-ray, which was found during pre-employment screening. She had no other complaints, and her past medical history was unremarkable. No fever, acute infectious symptoms and signs, or any other abnormalities were found in a physical examination.
Laboratory data showed a decrease in hemoglobin level (7.7 g/dL) with a normal mean corpuscular volume (88.9 fL) and platelet count (369×109/L). The diagnosis of iron-deficiency anemia was possible because the patient’s serum iron level (23 µg/dL) and serum transferrin saturation percentage (11.17%) had decreased, but total iron binding capacity (206 µg/dL) and her ferritin level (89.0 ng/mL) were normal, suggesting inflammatory anemia. Renal and hepatic function was normal. She was seronegative for HIV.
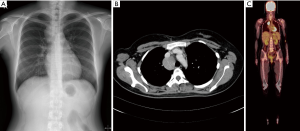
Chest X-ray and chest computed tomography (CT) confirmed a mass (6 cm × 4 cm × 4 cm) at the right superior and mid-mediastinum (Figure 1A,B). Positron emission tomography (PET) showed accumulation of fluorine-18-2-fluoro-2-deoxy-D-glucose in the tumor at a maximum standard uptake value of 9.21, whereas no other abnormal uptake suggestive of a metastatic lesion was found (Figure 1C). On the basis of the imaging findings, preoperative differential diagnoses included lymphoma, CD, thymoma, and neurogenic tumor. Diagnostic thoracoscopic surgery was planned based on the suspicion of lymphoma. A double-lumen endotracheal tube was placed, and the patient was positioned in the left lateral decubitus position. With single-lung ventilation, an initial 10-mm port was placed in the seventh intercostal space at the midaxillary line. This was followed by placement of two additional 10-mm ports in the fifth intercostal space at the anterior axillary line and the sixth intercostal space at the posterior axillary line.
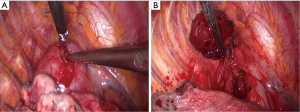
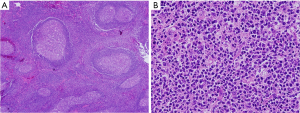
Thoracoscopic exploration revealed no other gross abnormalities, except for the mass located at superior mediastinum, which was located between the azygous and superior vena cava. After incising the parietal pleura, a large, highly vascular, soft tissue mass was observed (Figure 2A). Small pieces of tumor tissue were submitted for intraoperative pathological examination by frozen section. The pathological examination showed no evidence of malignancy and no diagnostic conclusion was made. One of the 10-mm ports at the anterior axillary line was converted to a 3-cm working port to remove the mass. During mobilization by dissection, we observed that the tumor was fragile and tended to bleed easily and there was loose adhesion between the mass and surrounding organs with a single feeding vessel at the center of the mass. However, meticulous bleeding control to avoid break down the mass by use of electrocautery and clips allowed this mass to be removed completely without damage to surrounding structures (Figure 2B, Figure 3). Macroscopic examination showed a 5.8 cm × 4.5 cm × 2.5-cm tumor weighing 36 g. Blood loss during surgery did not exceed 300 mL. A single chest tube was placed with achievement of hemostasis. The patient had the chest tube removed on the 4th postoperative day and was discharged home the next day. A pathological diagnosis was consistent with plasma cell type CD (Figure 4A,B). Three months after surgery, without any medical intervention, the laboratory data had recovered to the normal range (hemoglobin, 14.5 g/dL). She receives regular follow-ups and no recurrence has been found for 5 years since surgery.

Discussion
Unicentric CD of the mediastinum in patients with anemia has rarely been reported (6,7). CD, which is also known as angiofollicular giant lymph node hyperplasia, is an uncommon disease that causes benign lymph node hyperplasia, usually in the thorax. The cause of CD is still unclear (1,2).
The main histological subtypes of CD are the hyaline vascular type and the plasma cell type. The hyaline vascular type includes 80% to 90% of cases, and the plasma cell type includes the remaining 10% to 20% of cases (1,8). Clinically, CD is separated into unicentric or multicentric forms. In unicentric CD, the hyaline vascular type comprises up to 90% of cases and the plasma cell type comprises approximately 10-20% of cases. While the hyaline vascular type is rarely found in multicentric CD, 80-90% of multicentric CD cases are plasma cell type (9-11). Systemic manifestations, such as anemia, fever, excessive sweating, weight loss, night sweating, fatigue, and growth retardation are more common in multicentric CD, and are rarely reported in unicentric CD (1,3,8,12).
Anemia, which is an important feature of CD, is dominated by inflammatory or autoimmune hemolytic mechanisms, or both (12). Autoimmune hemolytic anemia is limited to the plasma cell type of multicentric CD. In unicentric CD, the usual situation is a young adult with chronic anemia who is unresponsive to iron therapy (12). In CD, it has been reported that the lymph nodes may excessively produce cytokines including interleukin-6 (IL-6). Anemia in CD is probably related to IL-6 and hepcidin, as in other chronic disease (8-10,12).
In unicentric CD, making the correct diagnosis is difficult before the operation. Unicentric CD is a rare discovery on radiographs. No clinical, radiological, or cytological features are relevant, and images are similar to those seen in lymphoma or thymoma. Diagnosis of unicentric CD is exclusively achieved with histological and immunohistochemical findings after resection (4,10). In this case, we were not aware of the possibility of CD or the association between anemia and the mediastinal mass in the absence of pathologic confirmation. Radiological evaluation mainly involves CT, MRI, and PET/CT. The typical feature on a CT scan is a well-circumscribed mass with soft tissue attenuation and calcification is rare (3,4). On MRI, these highly vascular tumors appear solid and have an intermediate to high signal compared with muscle on T1-weighted images. Hyperintense signals are seen on T2-weighted images (11). PET scans have been suggested for patients with multicentric CD to demonstrate the spread of lymphadenopathy and evaluate the response to treatment (13).
In unicentric CD, surgical resection of the mass is a curative method in 95% of patients, and constitutional symptoms may be resolved (8,9). However, there are few reports of thoracoscopic resections for CD because increased vascularization of CD causes excessive bleeding (14,15). Rena et al. suggested that thoracoscopic resection should be avoided because of its hypervascularity (16). Iyoda et al. reported conversion to open thoracotomy because of uncontrollable intraoperative bleeding (17). Preoperative embolization can be considered prior to surgical resection to minimize intraoperative bleeding (14,18).
In unicentric CD, radical radiotherapy is suitable for patients with locally advanced and inoperable disease, those unable to undergo surgery because of medical disorders, or those who refuse surgery. In general, radiotherapy of 40 to 50 Gy is necessary to achieve complete or partial remission (19,20). Postoperative radiotherapy for unicentric CD is recommended because of the possibility of relapse after partial excision (19,20). Recently, neoadjuvant radiotherapy enabled a successful radical resection (21). However, radiotherapy is associated with acute and late toxicity, as well as stricture of the esophagus, trachea, and bronchus (19,20).
In conclusion, mediastinal CD, although uncommon, should be included in the differential diagnosis of mediastinal tumors with anemia. Appropriate preparation for massive bleeding, such as preoperative embolization, would be helpful for safer thoracoscopic resection. The thoracoscopic approach of unicentric CD appears to be a safe and effective treatment modality, and constitutional symptoms may be resolved. The prognosis of this condition following complete resection appears to be good.
Acknowledgements
Funding: This study was supported by grants from the Catholic University of Korea, College of Medicine.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Dham A, Peterson BA. Castleman disease. Curr Opin Hematol 2007;14:354-9. [PubMed]
- Parez N, Bader-Meunier B, Roy CC, et al. Paediatric Castleman disease: report of seven cases and review of the literature. Eur J Pediatr 1999;158:631-7. [PubMed]
- Kim TJ, Han JK, Kim YH, et al. Castleman disease of the abdomen: imaging spectrum and clinicopathologic correlations. J Comput Assist Tomogr 2001;25:207-14. [PubMed]
- McAdams HP, Rosado-de-Christenson M, Fishback NF, et al. Castleman disease of the thorax: radiologic features with clinical and histopathologic correlation. Radiology 1998;209:221-8. [PubMed]
- Suh JH, Hong SH, Jeong SC, et al. Mediastinal tumor excision. Asvide 2015;2:070. Available online: http://www.asvide.com/articles/614
- Geary CG, Fox H. Giant lymph node hyperplasia of the mediastinum and refractory anaemia. J Clin Pathol 1978;31:757-60. [PubMed]
- Maier HC, Sommers SC. Mediastinal lymph node hyperplasia, hypergammaglobulinemia, and anemia. J Thorac Cardiovasc Surg 1980;79:860-3. [PubMed]
- Casper C. The aetiology and management of Castleman disease at 50 years: translating pathophysiology to patient care. Br J Haematol 2005;129:3-17. [PubMed]
- Beck JT, Hsu SM, Wijdenes J, et al. Brief report: alleviation of systemic manifestations of Castleman's disease by monoclonal anti-interleukin-6 antibody. N Engl J Med 1994;330:602-5. [PubMed]
- Arlet JB, Hermine O, Darnige L, et al. Iron-deficiency anemia in Castleman disease: implication of the interleukin 6/hepcidin pathway. Pediatrics 2010;126:e1608-12.
- Ecklund K, Hartnell GG. Mediastinal Castleman disease: MR and MRA features. J Thorac Imaging 1994;9:156-9. [PubMed]
- Vinzio S, Ciarloni L, Schlienger JL, et al. Isolated microcytic anemia disclosing a unicentric Castleman disease: The interleukin-6/hepcidin pathway? Eur J Intern Med 2008;19:367-9. [PubMed]
- Barker R, Kazmi F, Stebbing J, et al. FDG-PET/CT imaging in the management of HIV-associated multicentric Castleman’s disease. Eur J Nucl Med Mol Imaging 2009;36:648-52. [PubMed]
- Seirafi PA, Ferguson E, Edwards FH. Thoracoscopic resection of Castleman disease: case report and review. Chest 2003;123:280-2. [PubMed]
- Shetty S, Brenes RA, Panait L, et al. Video assisted thoracoscopic resection of a posterior mediastinal Castleman’s tumor. J Cardiothorac Surg 2011;6:113. [PubMed]
- Rena O, Casadio C, Maggi G. Castleman’s disease: unusual intrathoracic localization. Eur J Cardiothorac Surg 2001;19:519-21. [PubMed]
- Iyoda A, Yusa T, Hiroshima K, et al. Castleman’s disease in the posterior mediastinum: report of a case. Surg Today 2000;30:473-6. [PubMed]
- Robert JH, Sgourdos G, Kritikos N, et al. Preoperative embolization of hypervascular Castleman’s disease of the mediastinum. Cardiovasc Intervent Radiol 2008;31:186-8. [PubMed]
- Chronowski GM, Ha CS, Wilder RB, et al. Treatment of unicentric and multicentric Castleman disease and the role of radiotherapy. Cancer 2001;92:670-6. [PubMed]
- Neuhof D, Debus J. Outcome and late complications of radiotherapy in patients with unicentric Castleman disease. Acta Oncol 2006;45:1126-31. [PubMed]
- de Vries IA, van Acht MM, Demeyere T, et al. Neoadjuvant radiotherapy of primary irresectable unicentric Castleman’s disease: a case report and review of the literature. Radiat Oncol 2010;5:7. [PubMed]


