Extra Corporeal Membrane Oxygenation (ECMO) review of a lifesaving technology
Introduction
Extra Corporeal Membrane Oxygenation (ECMO) has remarkably progressed over the recent years; it became invaluable tool in the care of adults and children with severe cardiac and pulmonary dysfunction refractory to conventional management (1,2). Nowadays ECMO has become more reliable with improvement in equipment, and increased experience, which is reflected in improving results. The indications are extended to more prolonged use in intensive care unit, such as bridge to transplant, for both cardiac and lung transplant and support for lung resections in unstable patients (3-14). According to the Extracorporeal Life Support Organization (ESLO) registry, ECMO was used in over 5,000 cases in 2014 (15), this immense increase of patients treated with ECMO and the vast expansion to its indications raises ethical questions about choosing what patients should be treated with ECMO, and when the ECMO support should be stopped (16).
ECMO should only by performed by clinicians with training and experience in its initiation, maintenance, and discontinuation. ECMO is a supportive therapy rather than disease modifying treatment in itself; best results are obtained if we chose the right patient, the right type of ECMO and the right type of configuration (site, management and complication anticipation) (17). Indications, patient selection, technical aspects, complications, and impact of ECMO on clinical outcomes will be discussed here.
Evolution of ECMO
Kolff and Berk (18) in 1944 noted that blood became oxygenated as it passed through the cellophane chambers of their artificial kidney. This concept was applied in 1953 by Gibbon who used artificial oxygenation and perfusion support for the first successful open heart operation (19). In 1965, Rashkind and colleagues were the first to use a bubble oxygenator as support in a neonate dying of respiratory failure (20). In 1969, Dorson et al. reported the use of a membrane oxygenator for cardiopulmonary bypass in infants (21). In 1970, Baffes et al. (22) reported the successful use of extracorporeal membrane oxygenation as support in infants with congenital heart defects undergoing cardiac surgery.
Long-term ECMO as support for severe respiratory failure was first successfully used in 1972 in an adult patient with post-traumatic respiratory failure (23). Kolobow was developing a new membrane lung optimized for carbon dioxide (CO2) removal as a possible application in patients with chronic obstructive pulmonary disease (24). In 1975, Bartlett et al. reported the first successful use of ECMO in neonates with severe respiratory distress (25).
However, the enthusiasm decreased significantly when Morris et al. failed to show an outcome advantage of additional extracorporeal support when compared to conventional mechanical ventilatory support in adult respiratory distress syndrome (ARDS) patients in their randomized trial published at the beginning of the 1990s (26). Despite this lack of evidence, a few centers around Europe and in United States continued to provide veno-venous extracorporeal support with standard mechanical ventilation, in selected patients, usually as a last resort with encouraging results (27-29).
The usage of ECMO flourish after the publication of the CESAR trial, which clearly showed an improvement in the death rate and severe disability 6 months after randomization of patients with severe respiratory failure treated with extracorporeal support in an expert high-case-volume center compared with no specialized hospital care (3). Since then the ECMO support applications exploded and continue to progress.
Clinical indications and contraindications for institution of ECMO
ECMO is a form of cardiopulmonary life-support, where blood is drained from the vascular system, circulated outside the body by a mechanical pump, and then reinfused into the circulation. While outside the body, hemoglobin becomes fully saturated with oxygen and CO2 is removed. Oxygenation is determined by flow rate, and CO2 elimination can be controlled by adjusting the rate of countercurrent gas flow through the oxygenator (30).
Indications for ECMO can be divided into three categories according to the supported organ, cardiac, and respiratory support or a combination of the two. According to the data from the annual international ELSO Registry Reports through January 2015, over 65,171 patients received extracorporeal life support (ECLS) (15). The majority of patients were neonates 53%, 25% were pediatric and 23% were adults. The distribution of ECLS support included over 41,300 (63%) cases for respiratory support, over 18,700 (29%) cases for cardiac support and over 5,100 (8%) case for extracorporeal cardiopulmonary resuscitation (ECPR) (15).
Indications of ECMO for cardiac support
Typical cardiac indications include refractory low cardiac output (cardiac index less 2 L⁄min⁄m2) and hypotension (systolic blood pressure <90 mmHg) despite adequate intravascular volume, high dose inotropic agents and an intra-aortic balloon pump (17). The cardiac indications are summarized in Table 1.

Full table
Indications of ECMO for respiratory support
Both VV ECMO and VA ECMO can be used as a rescue therapy in acute respiratory failure to buy time and maintaining life awaiting improvement of the underlying disease. ECMO is used to provide oxygenation and CO2 removal, or both while the lungs recover, or as a bridge to transplant in case of end stage lung disease. The pulmonary indications are shown in Table 2.

Full table
Contraindications to ECMO: the contraindications to ECMO are shown in Table 3.

Full table
Techniques
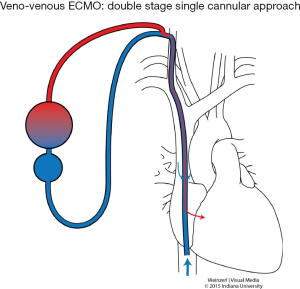
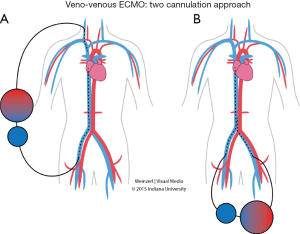
In VV ECMO, the patient must have stable hemodynamics. When single venous cannula is used, the blood is extracted from the vena cava or right atrium circulated and returned to the right atrium (Figure 1). The cannulae are usually placed percutaneously by Seldinger technique, via the right internal jugular vein. However if double venous cannula system is used; the cannulas are usually placed in the common femoral vein (for drainage) and right internal jugular or femoral vein (for infusion) (Figure 2).
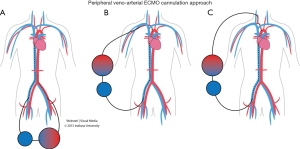
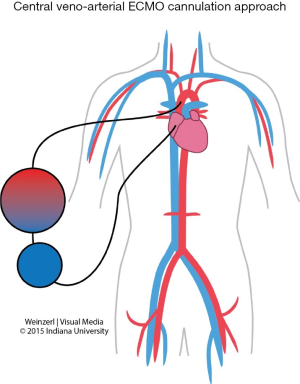
VA ECMO provides both respiratory and hemodynamic support; the ECMO circuit here is connected in parallel to the heart and lungs, while in VV ECMO the circuit is connected in series to the heart and lungs. During VA ECMO, blood will bypass both the heart and the lungs. Blood is extracted from the right atrium or vena cava (for drainage), and returned to the arterial system either through peripheral cannulations via femoral, axillary or carotid arteries (for infusion) (Figure 3) or into the ascending aorta if central cannulation is used, especially in cases of postcardiotomy ECMO where the cannulas employed for cardiopulmonary bypass can be transferred from the heart lung machine to the ECMO circuit. Blood is drained from the right atrium and reinfused into the ascending aorta (Figure 4). Comparison between VA and VV ECMO is shown in Table 4.

Full table
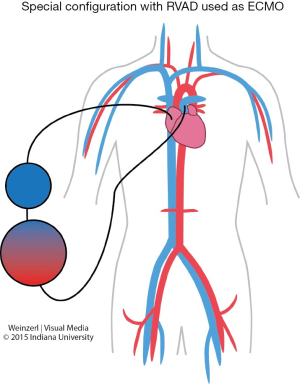
In special configuration when RVAD used as ECMO the oxygenated blood is delivered to the pulmonary artery so the blood bypass only the right heart (Figure 5).
Femoral access is preferred for VA ECMO in case of emergency or cardiogenic shock because insertion is relatively less invasive and faster to institute the ECMO. The probability of an ischemia of the ipsilateral lower extremity can be decreased by inserting an additional arterial cannula distal to the femoral artery cannula to perfuse the distal extremity at the time of ECMO insertion. Alternatively, a cannula can be inserted into the posterior tibial artery for retrograde flow to the extremity (31). If the patient is in cardiac cath lab or ICU and is too critical to go to the operating room for open insertion with end to side anastomosis with graft. Occasionally, the femoral vessels are unsuitable for cannulation for VA ECMO (e.g., patients with severe occlusive peripheral artery disease or prior femoral arterial reconstruction). In such circumstances, the right common carotid artery should be considered as alternative insertion site, this technique is associated with increased risk of a large watershed cerebral infarction 5-10%. One other alternative is using of the axillary artery, which offers the advantage of allowing patients on ECMO to ambulate (32,33).
Compare indications
The amount of oxygen provided via artificial lung is a directly related to the blood flow. The blood flow required during veno-venous approach to achieve acceptable arterial oxygenation is usually between 3 and 6 L/min. Arterial oxygenation is partially dependent on the cardiac output of the patient (as it is connected in series with the heart), and the hemoglobin concentration and saturation (34).
The veno-arterial approach implies the drainage of venous blood, its oxygenation and the subsequent input in the arterial system through a cannulated artery. The improvement of systemic oxygenation with this technique is much better as compared with the veno-venous approach because the artificially oxygenated blood mixes with arterial blood and directly perfuses distal organs. Moreover, in this type of cannulation the oxygenator is in parallel with the natural lung, comparison between VV and VA ECMO in Table 4.
ECMO complications
Complications on ECMO are very common and as expected it is associated with significant increase in morbidity and mortality. These complication could be related to the underlining pathology needed ECMO, or of the ECMO condition itself (surgical insertion, circuit tubing, anticoagulation etc.) and as a rule of thumb, the ECMO inserted for pulmonary support has less complication than the ECMO inserted for cardiogenic support. The worst outcomes are reported when ECMO is used after ECPR. VV ECMO has fewer complications than VA ECMO, the children have less complications than adults except for neurologic complications. The most frequent complication during ECMO is hemorrhage, ranging between 10-30% (35,36). Aubron et al. reported up to 34% in VA ECMO and 17% VV ECMO required surgery for bleeding issues (37), Bleeding may occur at the surgical site, at the cannula site, or into the site of a previous invasive procedure, intrathoracic, abdominal, or retroperitoneal hemorrhage may also occur. Bleeding is increased because of systemic heparinization, platelet dysfunction, and clotting factor hemodilution. Bleeding is managed by decreasing or stopping heparin and infusion of platelets and clotting factors (38). Infusion of activated factor VII has been reported with mixed results and should only be considered for life threatening hemorrhage after all other options have failed (39).
Pulmonary hemorrhage is seen commonly in patients on ECMO. Management includes bleeding control as above, using steroids, and frequent bronchoscopy to clear the airway over time.
Intracerebral hemorrhage or infarction occurs in approximately 10-15% of ARDS patients on ECMO. Forty-three percent of the deaths in the ECMO (40) series were associated with intracranial hemorrhage.
Hemolysis does not occur during ECMO unless there is a problem in the circuit or the patient. Plasma free hemoglobin should be checked frequently; values over 10 mg% requires further investigation in identifying and repairing the cause.
Systemic thromboembolism due to thrombus formation within the extracorporeal circuit, although it can be devastating, it is an infrequent complication. Its impact is greater with VA ECMO than VV ECMO because infusion is into the systemic circulation. Heparin infusion to achieve activated coagulation time (ACT) target, and vigilant observation of the circuit for signs of clot formation successfully prevents thromboembolism in most patients. Intracardiac thrombus formation (Figure 6) was also reported in literature (31).
Heparin-induced thrombocytopenia (HIT) can occur in patients receiving ECMO. When HIT is proven, heparin infusion should be replaced by a non-heparin anticoagulant. We favor using Bivalirudin in spite of Heparin even without HIT when anticipating long term ECMO use. Some authors prefer to use Argatroban because its half life is short and a similar ACT target range is effective (41).
Neurological complications are highly variable ranging between 4-37% (40,42) according to the ELSO registry (43) the incidence of major neurologic morbidity in cardiac patients as reported to ELSO is highest in neonates, with 7% suffering seizures, 3.5% infarction, and 11% intracranial hemorrhage. Children have slightly lower incidence of seizures and hemorrhage and a slightly higher incidence of infarction. Adults have the lowest incidence of major neurologic morbidity with 2% suffering seizures, 4% infarction, and 2% hemorrhage. In all age groups those patients who suffer major neurologic complications have a lower hospital survival (43). In Lidegran et al. (44) series of 123 patients underwent ECMO a head CT scan was performed on 87 patients, the CT scan findings were Seventy-eight patients had cranial CT while on ECMO. Inntra cranial hemorrhage (ICH) or cerebral infarction was detected in 37%, 15% had focal hemorrhage, 9% focal infarction, and 13% general brain edema. The CT findings were decisive in 16 of the 45 patients to withdraw the ECMO treatment. It is important to realize that these findings may be a consequence of the condition that prompted ECMO, rather than a complication of the ECMO process (42). These may be partially due to systemic heparinization, thrombocytopenia, coagulopathies, or systolic hypertension.
Medical complications
Hypertension is a dangerous complication because of the risk of hemorrhage and stroke. Arrhythmias may occur as a result of hypoxia and electrolyte imbalance or an underlining cardiac pathology. Oliguria is a commonly observed renal complication during the early part of ECMO; acute tubular necrosis is observed in some patients and may require hemofiltration and dialysis. GI tract complications include hemorrhage (44), which may occur as a result of stress, ischemia, or bleeding tendencies. Direct hyperbilirubinemia and biliary calculi may occur secondary to prolonged fasting and total parenteral nutrition (TPN), hemolysis, and diuretics.
Septic complications may also result because the ECMO circuit represents a large intravascular foreign body, and frequent manipulation increases the risk of infection. Metabolic complications include electrolyte imbalances, and hypo or hyperglycemia. ECMO may alter serum concentration of drugs due to increased volume of distribution, and decreased Kidney or liver function. Caution is warranted when narrow therapeutic drugs are administered, and dose alterations may be necessary (45).
Mechanical complications: clots in the circuit are the most common mechanical complication (19%). Major clots can cause oxygenator failure, consumption coagulopathy, and pulmonary or systemic emboli. More recently, heparin-coated ECMO systems have been used to decrease the frequency of this complication.
VA ECMO-specific complications
Cannulation related complications: a variety of complications can occur during cannulation, including vessel perforation with hemorrhage, arterial dissection, distal ischemia, and incorrect location (e.g., venous cannula within the artery) or development of pseudoaneurysm at the site of insertion. These complications are rare <5%. A skilled and experienced surgeon is important to avoid or address such complications.
Cardiac thrombosis (Figure 6): occurs secondary to retrograde blood flow in the ascending aorta whenever peripheral cannulation through the femoral artery and vein are used for VA ECMO. Stasis of the blood can occur if left ventricular output is not maintained, which may result in cardiac thrombosis.
Coronary or cerebral hypoxia: during VA ECMO, fully saturated blood infused peripherally into the femoral artery from the ECMO circuit will preferentially perfuse the lower extremities and the abdominal viscera. Blood ejected from the heart will selectively perfuse the heart, brain, and upper extremities. As a result, cardiac and cerebral hypoxia could exist and be unrecognized if oxygenation is monitored using only blood from the lower extremity. To avoid this complication, arterial oxyhemoglobin saturation should be monitored in both the upper extremity and the lower extremity. Poor arterial oxyhemoglobin saturations measured from the upper extremity is corrected by infusing some oxygenated blood into the right atrium.
Organ management in ECMO patient
Organ management is very critical in obtaining good survival outcomes with better quality of life, better general health, physical health, and social functioning. The aim of organ management is to avoid multiorgan failure in a patient who is suffering primary of the failure of the heart, lung or both; before initiating the ECMO, better understanding to the new hemodynamics and physiologic body response to the ECMO circuit and hemodynamics play a major role in outcomes.
Cardiovascular system management: systemic perfusion and intravascular volume should be maintained. Volume status can be assessed clinically by urine output, central venous pressure, physical signs of perfusion, and body weight. Good cardiac output should be achieved with inotropic agents. Echocardiography should be performed to assess the heart’s condition, and heart recovery, rule out thrombosis, and should be repeated if any significant changes in the ECMO flow, or deterioration in the patient’s hemodynamics.
Pulmonary system management: ECMO is used temporarily while awaiting lung recovery. In some centers, a high PEEP has been used to avoid atelectasis. Pulmonary hygiene is strict and requires frequent positional changes, endotracheal suctioning every 4 hours depending on secretions, a daily chest radiograph, and flexible bronchoscopy when needed.
Renal system management: during the first 24-48 hours ECMO patients experience an oliguric phase as the ECMO circuit triggers an acute inflammatory reaction. This leads to capillary leak and intravascular volume depletion resulting in oliguria and acute tubular necrosis. After 48 hours the diuretic phase begins (which is one of the earliest signs of recovery). If oliguria persists for 48-72 hours, diuretics are often required to reduce edema. When renal failure does not improve, continuous renal replacement therapy (CRRT) may be added to the circuit. Kielstein et al. (46) demonstrated that 60% of ECMO patients required CRRT for acute renal injury (AKI), the 3-month survival of ECMO patients requiring CRRT for AKI was 17% vs. 53% without CRRT. They concluded that AKI requiring CRRT in patients undergoing ECMO treatment increases mortality and the longer duration of CRRT was also associated with a higher mortality. Zwiers et al. (47) showed that neonates who suffer AKI in association with ECMO are at increased risk for developing chronic kidney disease (CKD) and/or hypertension in median of 8.2 years of follow-up.
Central nervous system (CNS) managements: CNS complications are very common; it was reported in up to 48% of patients who received ECMO for greater than 12 hours (3). These complications are related to the degree of hypoxia and acidosis or primarily as consequence of the condition that prompted ECMO, rather than a complication of the ECMO process. Avoiding paralytic agents and performing regular sedation vacation and neurologic examinations are recommended. Imaging studies though difficult to obtain in unstable patients should be used in case of suspicion (44). In patients with seizures or suspected seizures, aggressive treatment is recommended.
Infection control: strict aseptic precautions are required (13). The presence of infection is monitored by obtaining cultures from the circuit at least once a week or when infection is suspected. Based on institutional experience, the protocol frequency may vary.
Hematologic considerations: to optimize oxygen delivery, the patient’s hemoglobin should be maintained at higher than 8 g/dL. As a result of platelet consumption during ECMO, platelet transfusions are required to maintain platelet counts above 100,000/mcL. Activated clotting time (ACT) should be maintained at 180-240 seconds to avoid bleeding complications (48).
We favor using direct thrombin inhibitors like Bivalirudin in spite of Heparin when anticipating long term ECMO use as, LVAD or transplant candidates. Bivalirudin dose adjustments are needed to maintain APTT 1.5-2.5 times baseline values or within the physician-defined range (49). Some authors prefer to use Argatroban because its half life is shorter than heparin and a similar ACT target range is effective.
Fluids, Electrolytes and Nutrition: patients on ECMO require close monitoring of fluids and electrolytes (potassium, magnesium, phosphorus and ionized calcium). The high-energy requirements should be met using hyperalimentation techniques. The patient’s weight increases in the first 1-3 days on ECMO because of the need of fluid resuscitation, fluid retention, and oliguric phase.
Outcomes
According to the data from the annual international ELSO Registry Reports through January 2015 (15), 65,171 patients received ECLS, among these, 71% were weaned and 59% were discharged or transferred. Use of ECLS for respiratory support represents a large area of consistent growth. Over 141,300 patients have been treated with survival to discharge rates of 74%, 57%, and 57% for neonates, pediatric, and adults, respectively. Use of ECLS for cardiac support also represents a large area of consistent growth. Approximately 18,800 patients have been treated ECLS with survival to discharge rates of 41%, 50%, and 41% for neonates, pediatric, and adults, respectively (15).
The survival of patients undergoing ECMO can be categorized according to the indication for the ECMO: severe acute respiratory failure or cardiac failure.
Acute respiratory distress syndrome (ARDS): Hemmila et al. (36) showed that 67% of patients with acute respiratory failure treated with ECMO were weaned off ECMO and 52% survived to hospital discharge. This was also confirmed by the CESAR study (3) which demonstrated that referral to an ECMO center significantly improves recovery and survival from severe ARDS. Therefore, we recommend that adult patients with severe ARDS be referred to an ECMO center. In experienced ECMO centers, approximately 25% of patients will improve and recover without ECMO, while 75% of patients will require ECMO. Among those who require ECMO, 60% to 70% will survive. The group referred to the ECMO center had significantly increased survival without disability at six months compared to conventional management (63% vs. 47%). Similar conclusions were obtained regarding referral and transfer patients with severe influenza H1N1-related acute respiratory distress syndrome to an ECMO center, this was associated with lower hospital mortality (23.7% vs. 52.5%) (50).
Venoarterial (VA) ECMO can provide acute support in cardiogenic shock or cardiac arrest in adults. Assuming that the brain function is normal or only minimally impaired, ECMO is provided until the patient recovers or receives a long-term ventricular assist device as a bridge to cardiac transplantation. Survival rate reported in the literature range between 20-30% among patients who received VA ECMO for cardiac arrest, severe cardiogenic shock, or failure to wean from cardiopulmonary bypass following cardiac surgery (13,51,52). Shin et al. (53) demonstrated that ECMO performed for cardiac arrest was associated with increased survival with minimal neurologic impairment compared to conventional cardiopulmonary resuscitation, these findings were confirmed by Chen et al. at discharge time, 30 days, and 1 year survival (54).
Safeguard and tips for safe technique
VV ECMO: the perfect position when using one venous cannula is when the distal tip of the cannula lying below the diaphragm (in the IVC) with the proximal opening in the SVC or SVC/right atrium junction, orienting the opening of the arterial cannula toward the tricuspid valve (Figure 1). Check cannula position regularly and on a daily basis, and during acute events in such as significant drop in ECMO flow, or in blood saturation. Immigration into the right ventricle or into the coronary sinus should be ruled out. And when signs of right ventricle dysfunction manifest we should consider switching to VA ECMO.
Peripheral VA ECMO: should be avoided in a patient with a history of peripheral vascular disease, if Seldinger technique is used; avoid to put the arterial and venous cannula in the same groin side, to avoid venous congestion from the collapsed femoral vein laminated by the adjacent arterial cannula, insert only the tip of the arterial cannula, the best way to avoid distal ischemia is to insert the arterial cannula on end to side graft (the cannula is not in the artery lumen), don’t wait for compartment syndrome signs to develop, to perform fasciotomy. Be aware of the risk of left ventricle dysfunction and distension in case of low LV function because of poorly contracting, non-ejecting left ventricle or atrium as little blood reaches the left atrium with good right atrial drainage, switch the peripheral cannulation to central with LV vent, keep the patient on inotropic drugs such as Epinephrine, Dobutamine or Norepinephrine, adding peripheral impeller, or percutaneous atrial septostomy represent alternative options (55,56).
In case of central VA ECMO in case of a patient with LV dysfunction; add LV vent to the circuit and proceed as mentioned earlier, insert the cannulas in separate incision of the surgical incision to temporarily close the chest this technique will help patient to resume ambulation.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Financial Disclosure: All Authors.
References
- MacLaren G, Combes A, Bartlett RH. Contemporary extracorporeal membrane oxygenation for adult respiratory failure: life support in the new era. Intensive Care Med 2012;38:210-20. [PubMed]
- Shekar K, Mullany DV, Thomson B, et al. Extracorporeal life support devices and strategies for management of acute cardiorespiratory failure in adult patients: a comprehensive review. Crit Care 2014;18:219. [PubMed]
- Peek GJ, Mugford M, Tiruvoipati R, et al. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet 2009;374:1351-63. [PubMed]
- Cooper DS, Jacobs JP, Moore L, et al. Cardiac extracorporeal life support: state of the art in 2007. Cardiol Young 2007;17 Suppl 2:104-15. [PubMed]
- Marasco SF, Esmore DS, Negri J, et al. Early institution of mechanical support improves outcomes in primary cardiac allograft failure. J Heart Lung Transplant 2005;24:2037-42. [PubMed]
- Acker MA. Mechanical circulatory support for patients with acute-fulminant myocarditis.Ann Thorac Surg 2001;71:S73-6; discussion S82-5.
- Clark JB, Pauliks LB, Myers JL, et al. Mechanical circulatory support for end-stage heart failure in repaired and palliated congenital heart disease. Curr Cardiol Rev 2011;7:102-9. [PubMed]
- Rinieri P, Peillon C, Bessou JP, et al. National review of use of extracorporeal membrane oxygenation as respiratory support in thoracic surgery excluding lung transplantation. Eur J Cardiothorac Surg 2015;47:87-94. [PubMed]
- McFadden PM, Greene CL. The evolution of intraoperative support in lung transplantation: Cardiopulmonary bypass to extracorporeal membrane oxygenation. J Thorac Cardiovasc Surg 2015;149:1158-60. [PubMed]
- Gulack BC, Hirji SA, Hartwig MG. Bridge to lung transplantation and rescue post-transplant: the expanding role of extracorporeal membrane oxygenation. J Thorac Dis 2014;6:1070-9. [PubMed]
- Bermudez CA, Shiose A, Esper SA, et al. Outcomes of intraoperative venoarterial extracorporeal membrane oxygenation versus cardiopulmonary bypass during lung transplantation. Ann Thorac Surg 2014;98:1936-42; discussion 1942-3.
- Pham T, Combes A, Rozé H, et al. Extracorporeal membrane oxygenation for pandemic influenza A(H1N1)-induced acute respiratory distress syndrome: a cohort study and propensity-matched analysis. Am J Respir Crit Care Med 2013;187:276-85. [PubMed]
- Combes A, Leprince P, Luyt CE, et al. Outcomes and long-term quality-of-life of patients supported by extracorporeal membrane oxygenation for refractory cardiogenic shock. Crit Care Med 2008;36:1404-11. [PubMed]
- Bréchot N, Luyt CE, Schmidt M, et al. Venoarterial extracorporeal membrane oxygenation support for refractory cardiovascular dysfunction during severe bacterial septic shock. Crit Care Med 2013;41:1616-26. [PubMed]
- Extracorporeal Life Support Registry Report. Available online: https://www.elso.org/Registry/Statistics/InternationalSummary.aspx, accessed on February 15 2015.
- Ramanathan K, Cove ME, Caleb MG, et al. Ethical dilemmas of adult ECMO: emerging conceptual challenges. J Cardiothorac Vasc Anesth 2015;29:229-33. [PubMed]
- Fraser JF, Shekar K, Diab S, et al. ECMO – the clinician’s view. ISBT Sci Ser 2012;7:82-8.
- Kolff WJ, Berk HT, ter Welle M, et al. The artificial kidney: a dialyser with a great area. 1944. J Am Soc Nephrol 1997;8:1959-65. [PubMed]
- Gibbon JH Jr. Application of a mechanical heart and lung apparatus to cardiac surgery. Minn Med 1954;37:171-85. [PubMed]
- Rashkind WJ, Freeman A, Klein D, et al. Evaluation of a disposable plastic, low volume, pumpless oxygenator as a lung substitute. J Pediatr 1965;66:94-102. [PubMed]
- Dorson W Jr, Baker E, Cohen ML, et al. A perfusion system for infants. Trans Am Soc Artif Intern Organs 1969;15:155-60. [PubMed]
- Baffes TG, Fridman JL, Bicoff JP, et al. Extracorporeal circulation for support of palliative cardiac surgery in infants. Ann Thorac Surg 1970;10:354-63. [PubMed]
- Hill JD, O'Brien TG, Murray JJ, et al. Prolonged extracorporeal oxygenation for acute post-traumatic respiratory failure (shock-lung syndrome). Use of the Bramson membrane lung. N Engl J Med 1972;286:629-34. [PubMed]
- Kolobow T, Gattinoni L, Tomlinson T, et al. The carbon dioxide membrane lung (CDML): a new concept. Trans Am Soc Artif Intern Organs 1977;23:17-21. [PubMed]
- Bartlett RH, Gazzaniga AB, Jefferies MR, et al. Extracorporeal membrane oxygenation (ECMO) cardiopulmonary support in infancy. Trans Am Soc Artif Intern Organs 1976;22:80-93. [PubMed]
- Morris AH, Wallace CJ, Menlove RL, et al. Randomized clinical trial of pressure-controlled inverse ratio ventilation and extracorporeal CO2 removal for adult respiratory distress syndrome. Am J Respir Crit Care Med 1994;149:295-305. [PubMed]
- Lewandowski K, Rossaint R, Pappert D, et al. High survival rate in 122 ARDS patients managed according to a clinical algorithm including extracorporeal membrane oxygenation. Intensive Care Med 1997;23:819-35. [PubMed]
- Kolla S, Awad SS, Rich PB, et al. Extracorporeal life support for 100 adult patients with severe respiratory failure. Ann Surg 1997;226:544-64; discussion 565-6. [PubMed]
- Bartlett RH, Roloff DW, Custer JR, et al. Extracorporeal life support: the University of Michigan experience. JAMA 2000;283:904-8. [PubMed]
- Schmidt M, Tachon G, Devilliers C, et al. Blood oxygenation and decarboxylation determinants during venovenous ECMO for respiratory failure in adults. Intensive Care Med 2013;39:838-46. [PubMed]
- Madershahian N, Nagib R, Wippermann J, et al. A simple technique of distal limb perfusion during prolonged femoro-femoral cannulation. J Card Surg 2006;21:168-9. [PubMed]
- Navia JL, Atik FA, Beyer EA, et al. Extracorporeal membrane oxygenation with right axillary artery perfusion. Ann Thorac Surg 2005;79:2163-5. [PubMed]
- Moazami N, Moon MR, Lawton JS, et al. Axillary artery cannulation for extracorporeal membrane oxygenator support in adults: an approach to minimize complications. J Thorac Cardiovasc Surg 2003;126:2097-8. [PubMed]
- Gattinoni L, Carlesso E, Langer T. Clinical review: Extracorporeal membrane oxygenation. Crit Care 2011;15:243. [PubMed]
- Bartlett RH, Gattinoni L. Current status of extracorporeal life support (ECMO) for cardiopulmonary failure. Minerva Anestesiol 2010;76:534-40. [PubMed]
- Hemmila MR, Rowe SA, Boules TN, et al. Extracorporeal life support for severe acute respiratory distress syndrome in adults. Ann Surg 2004;240:595-605; discussion 605-7. [PubMed]
- Aubron C, Cheng AC, Pilcher D, et al. Factors associated with outcomes of patients on extracorporeal membrane oxygenation support: a 5-year cohort study. Crit Care 2013;17:R73. [PubMed]
- Peek G, Wittenstein B, Harvey C, et al. Management of bleeding during ECLS. In: Van Meurs K, Lally KP, Peek G, et al. editors. ECMO in Critical Care. Extracorporeal life support organization, Ann Arbor 2005.
- Wittenstein B, Ng C, Ravn H, et al. Recombinant factor VII for severe bleeding during extracorporeal membrane oxygenation following open heart surgery. Pediatr Crit Care Med 2005;6:473-6. [PubMed]
- Australia and New Zealand Extracorporeal Membrane Oxygenation (ANZ ECMO) Influenza Investigators, Davies A, Jones D, et al. Extracorporeal Membrane Oxygenation for 2009 Influenza A(H1N1) Acute Respiratory Distress Syndrome. JAMA 2009;302:1888-95. [PubMed]
- Cornell T, Wyrick P, Fleming G, et al. A case series describing the use of argatroban in patients on extracorporeal circulation. ASAIO J 2007;53:460-3. [PubMed]
- Mateen FJ, Muralidharan R, Shinohara RT, et al. Neurological injury in adults treated with extracorporeal membrane oxygenation. Arch Neurol 2011;68:1543-9. [PubMed]
- Mehta A, Ibsen LM. Neurologic complications and neurodevelopmental outcome with extracorporeal life support. World J Crit Care Med 2013;2:40-7. [PubMed]
- Lidegran MK, Mosskin M, Ringertz HG, et al. Cranial CT for diagnosis of intracranial complications in adult and pediatric patients during ECMO: Clinical benefits in diagnosis and treatment. Acad Radiol 2007;14:62-71. [PubMed]
- Shekar K, Fraser JF, Smith MT, et al. Pharmacokinetic changes in patients receiving extracorporeal membrane oxygenation. J Crit Care 2012;27:741.e9-18.
- Kielstein JT, Heiden AM, Beutel G, et al. Renal function and survival in 200 patients undergoing ECMO therapy. Nephrol Dial Transplant 2013;28:86-90. [PubMed]
- Zwiers AJ, IJsselstijn H, van Rosmalen J, et al. CKD and hypertension during long-term follow-up in children and adolescents previously treated with extracorporeal membrane oxygenation. Clin J Am Soc Nephrol 2014;9:2070-8. [PubMed]
- ELSO Anticoagulation Guidelines 2014. Extracorporeal Life Support Organization. Available online: http://www.elso.org/Portals/0/Files/elsoanticoagulationguideline8-2014-table-contents.pdf, accessed February 15th 2015.
- Ranucci M, Ballotta A, Kandil H, et al. Bivalirudin-based versus conventional heparin anticoagulation for postcardiotomy extracorporeal membrane oxygenation. Crit Care 2011;15:R275. [PubMed]
- Noah MA, Peek GJ, Finney SJ, et al. Referral to an extracorporeal membrane oxygenation center and mortality among patients with severe 2009 influenza A(H1N1). JAMA 2011;306:1659-68. [PubMed]
- Smedira NG, Blackstone EH. Postcardiotomy mechanical support: risk factors and outcomes. Ann Thorac Surg 2001;71:S60-6; discussion S82-5.
- Elsharkawy HA, Li L, Esa WA, et al. Outcome in patients who require venoarterial extracorporeal membrane oxygenation support after cardiac surgery. J Cardiothorac Vasc Anesth 2010;24:946-51. [PubMed]
- Shin TG, Choi JH, Jo IJ, et al. Extracorporeal cardiopulmonary resuscitation in patients with inhospital cardiac arrest: A comparison with conventional cardiopulmonary resuscitation. Crit Care Med 2011;39:1-7. [PubMed]
- Chen YS, Lin JW, Yu HY, et al. Cardiopulmonary resuscitation with assisted extracorporeal life-support versus conventional cardiopulmonary resuscitation in adults with in-hospital cardiac arrest: an observational study and propensity analysis. Lancet 2008;372:554-61. [PubMed]
- Koenig PR, Ralston MA, Kimball TR, et al. Balloon atrial septostomy for left ventricular decompression in patients receiving extracorporeal membrane oxygenation for myocardial failure. J Pediatr 1993;122:S95-9. [PubMed]
- Aiyagari RM, Rocchini AP, Remenapp RT, et al. Decompression of the left atrium during extracorporeal membrane oxygenation using a transseptal cannula incorporated into the circuit. Crit Care Med 2006;34:2603-6. [PubMed]