Mediastinoscopic esophagectomy for patients with early esophageal cancer
Introduction
Esophageal cancer ranks sixth among all cancers in mortality and is one of the least studied and deadliest cancers worldwide because of its extremely aggressive nature and poor survival rate (1-3). The overall 5-year relative survival is 17% (4-6). Surgical resection remains the mainstream treatment for esophageal cancer (7). With an attempt to minimize the surgical trauma, resection of esophageal cancer under thoracoscope and/or laparoscope has become a standard procedure. On the premise of ensuring the good prognosis, the minimally invasive esophagectomy has remarkably lowered post-operative morbidity and mortality (8,9). However, thoracoscopic esophagectomy does not change the route of traditional open surgery and can still cause a similar surgical trauma within thoracic cavity; thus, it has limited benefits for high-risk patients (10). Resection of esophageal cancer via the diaphragm hiatus without routine open thoracotomy is more feasible for high-risk patients with serious comorbidities by significantly decreasing post-operative morbidity and mortality (11). However, it does not allow mediastinal lymphadenectomy. In contrast, the mediastinoscopic esophagectomy can both maintain the integrity of the pleural cavity and enable mediastinal lymphadenectomy; with smaller surgical trauma, it has relatively lower requirements on the patients’ cardiopulmonary functions (12,13). Currently, this procedure is only performed in a limited number of centers; it remains controversial compared with conventional thoracoscopic esophagectomy for esophageal cancer (10,14,15). The purpose of this study was to detect the feasibility, safety, and effectiveness of mediastinoscopic esophagectomy for early esophageal cancer.
Patients and methods
The study was approved by the Ethics Committee of Changzhou First People’s Hospital. A total of 194 esophageal cancer patients [128 men and 66 women aged 42-84 years (median: 62 years)] who underwent mediastinoscopic esophagectomy in our center from December 2005 to October 2014 were enrolled in this study. The disease was confirmed to be esophageal squamous cell carcinoma by endoscopy. Patients who were confirmed to be in stages cT1-2N0M0 by chest and abdominal computed tomography (CT) and endoscopic ultrasonography were selected. All the selected patients had no obvious cervical spine disease and their head and neck could be tilted back. The pre-operative physical examination, laboratory tests, ECG and lung function tests showed no significant abnormality.
All patients were in a supine position, with their back padded and heads tilted. Endotracheal intubation using a corrugated tube was performed under the general anesthesia. For the neck surgery, an incision was made along the left anterior sternocleidomastoid edge (up to the midpoint of the sternocleidomastoid and down to the jugular notch, about 6cm long). After the dissection along the anterior border of sternocleidomastoid muscle via the incision, part of the cervical esophagus was isolated under direct vision, followed by the insertion of mediastinoscope and isolation of thoracic esophagus. The esophagus was then dissociated forwards, backwards, leftwards, and rightwards. The nutritional support branch from the aorta for the esophagus was clipped using a titanium clip (or other hemostasis devices such as Harmonic and Ligasure) down to the level of the pulmonary veins. Meanwhile, the paraesophageal mediastinal lymph nodes were dissected. For patients with swollen subcarinal lymph nodes, the samples were collected using lymph node forceps for pathology. The stomach was dissociated via laparoscope or an anteromedian abdominal incision. Also, the lower esophagus was divided through the diaphragmatic hiatus till the level of inferior pulmonary vein. Gauze was placed as a marker, so as to ensure the esophagus was completely isolated. After the complete isolation of the esophagus and stomach, divide the cardia and then pull out the esophagus from the cervical incision. Enlarge the esophageal hiatus to send the gastric body to the neck along the esophageal bed for cervical gastroesophageal anastomosis. After the surgery, the patient was routinely sent to the intensive care unit for further monitoring and treatment, during which intravenous administration of morphine via micro pump was applied.
With the first post-operative year, the patient was arranged to receive follow-up visit at the outpatient departments every three months, followed by outpatient visit every six months, during which chest CT, neck/abdominal ultrasonography, and tests for tumor markers were performed to rule out any tumor relapse/metastasis. In case the patient did not visit the outpatient department timely, the doctor-in-charge would call the patient to urge him/her to receive the follow-up and learn the survival conditions. All the re-examination and follow-up results were entered into a medical record database. The peri-operative data and follow-up data were retrieved from the database to calculate the overall survival rate and survival time.
Statistical analysis
Data were analyzed using the GraphPad Prism 5.0 software. Data between the two groups were compared using Mann Whitney test, and survival analysis was conducted using Log-rank test. P<0.05 was considered significantly different.
Results
Mediastinoscopic esophagectomy was successfully performed in 193 patients (99.5%). In one patient, a small incision was made in the right chest for suture and hemostasis due to the uncontrollable intra-operative bleeding under the mediastinoscope. The average duration of thoracic surgery was 48.2±7.8 min and the average intra-operative blood loss was 128.1±34.5 mL. An average of 3.1±1.6 lymph node stations were dissected, with an average number of dissected lymph nodes being 9.38±6.2, among which 4.2±5.4 were mediastinal lymph nodes. No peri-operative death was noted, and the surgery-associated complication rate was only 13.4% (26/194). These complications included anastomotic leak (n=9, 4.6%), pneumonia (n=12, 6.2%), arrhythmia (n=7, 3.6%), hoarseness (n=9, 4.6%), and mediastinal celiac disease (n=1, 0.5%). All these patients were smoothly discharged after symptomatic treatment.
The post-operative pathology confirmed that 65 patients (33.5%) were in stage T1a, 94 (48.5%) in stage T1b, and 35 (18.0%) in stage T2 (Table 1). The differences in age and gender showed no statistical significance among patients with different T stages. The average number of the dissected lymph nodes and the positive rate of the dissected lymph nodes were significantly different. However, the thoracic operative time, intra-operative blood loss, number of dissected lymph node stations, number of dissected mediastinal lymph nodes, and the incidences of the complications were not significantly different.
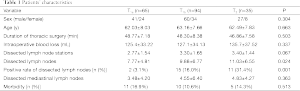
Full table
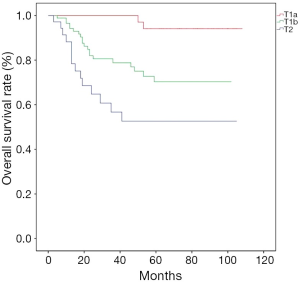
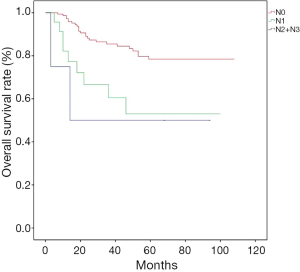
Patients were followed up for a median of 39 [3-108] months, and the overall 5-year survival was 72.73%. Till the end of follow-up, 53 patients died, with the death causes including tumor relapse/metastasis (n=48), pulmonary heart disease (n=1), and unknown causes (n=4). The overall survival rates significantly differed among different T stages (Figure 1); more specifically, the 5-year survival was 95.23% in patients with stage T1a esophageal cancer, 70.15% for T1b, and 55.56% for T2 (P<0.001). The overall survival was significantly better in patients with negative lymph nodes (N0) than those with lymph node metastasis (N1 and N2 + N3) (P=0.003, Figure 2), while no such significant difference was noted between N1 and N2 + N3 (P=0.696); more specifically, the 5-year survival rate was 84.9% for N0, 62.5% for N1, and 50.0% for N2 + N3.
Discussion
Of these patients who underwent mediastinoscopic esophagectomy, the average duration of thoracic surgery was 48.2±7.8 min and the average intra-operative blood loss was 128.1±34.5 mL. An average of 3.1±1.6 lymph node stations were dissected, with an average number of dissected lymph nodes being 9.38±6.2, among which 4.2±5.4 were mediastinal lymph nodes. No peri-operative death was noted, and the incidence of peri-operative complications was 13.4%. The overall survival significantly differed among patients with different T stages (P=0.001), and the overall survival was significantly better in patients with negative lymph nodes than those with lymph node metastasis (P=0.003).
The mediastinoscopic esophagectomy was initially applied for the resection of esophageal cancer in highly risk patients with serious comorbidities (15). Along with advances in minimally invasive surgical instruments and mediastinoscopic surgical procedures, the treatment of esophageal cancer via the mediastinal route has been widely applied (12,16), with satisfactory peri-operative effectiveness (13). However, the indications and long-term effectiveness of this procedure remain controversial (10,17). In our current study, by retrospectively analyzing the clinical data of 194 patients who underwent mediastinoscopic esophagectomy for early esophageal cancer, we tried to further clarify its long-term effectiveness.
During the surgery, two groups of medical staff were assigned to carry out cervical and abdominal operation simultaneously, which dramatically decreased the surgical time. This approach avoids thoracotomy and thus maintains the intact pleural cavity and minimizes surgical trauma. Notably, the operation on the neck during the procedure may easily cause the injury of recurrent laryngeal nerve. In our series, nine patients suffered from hoarseness after the surgery, which was restored after symptomatic treatment. In our experience, the use of suction cautery is associated with a high likelihood of recurrent laryngeal nerve injury. Therefore, when separating the upper esophagus for the later patients, the power system should be avoided and changed to Ligasure for the operation. Also, the postoperative morbidity in our series was 13.4%, which was lower than those reported in the conventional thoracoscopic surgery for esophageal cancer (7).
Lymph nodes are an independent risk factor of the prognosis of esophageal cancer. In our series, the pre-operative judgment was that there was no lymph node metastasis for all the 194 patients; however, lymph node metastasis was detected in 28 patients by the post-operative pathology, yielding an average positive rate of 14.95%. In our experiences, the amplification effect of mediastinoscopy is conducive to fine operation and thus help to achieve the complete resection of the enlarged mediastinal lymph nodes under direct vision during the operation (12,18). However, due to the limitations in the angles of surgical instruments, it is technically unable to dissect the subcarinal lymph nodes via the mediastinal route. In our current series, 82 patients were observed enlarged subcarinal lymph nodes during the operation. Sampling with lymph node biopsy forceps showed negative results. However, metastases to the cervical or peri-gastric lymph nodes were confirmed in 24 patients by post-operative pathology. As described in previous literature, lymphatic drainage of thoracic esophagus is basically longitudinal; thus, subcarinal lymph node metastasis was less common in patients with early esophageal cancer (19).
In our series, the overall 5-year survival was 72.73%, and it significantly differed among patients with different T stages. Meanwhile, the overall survival rate was significantly higher in patients with negative lymph node than those with lymph node metastasis, which was consistent with the findings in patients who underwent conventional thoracoscopic surgery (20). In our series, the overall survival rate of patients with negative lymph node reached 84.9%, which was equal to or even superior to the survival rates reported in literature on the conventional thoracoscopic surgery, suggesting that the mediastinoscopic route have similar effectiveness in treating the early esophageal cancer to the conventional thoracoscopic surgery. However, the survival rate remarkably decreased in patients with lymph node metastasis and was inferior to that in patients who underwent the conventional thoracoscopic surgery; thus, the pre-operative assessment (e.g., PET-CT) should be further enhanced before the mediastinoscopic esophagectomy. Meanwhile, repeated intraoperative rapid frozen section is required. For patients who were found to be with lymph node metastasis during the intra-operative frozen section, transfer to a conventional thoracoscopic surgery should be arranged if the general condition allows, which might benefit parts of patients with lymph node metastasis.
This study also had its limitations. First, it was a retrospective clinical study. Nevertheless, we tried our best to summarize all the available clinical characteristics in our clinical database; meanwhile, the data were reviewed by senior doctors to ensure their accuracies. Second, in our center there was no corresponding data on the clinical effectiveness of conventional thoracoscopic surgery. Instead, we did a massive literature review to identify the recent advances in thoracoscopic techniques and their long-term clinical effectiveness, and the results were used for the comparison with the data in our own series. The clinical data were reviewed again, when necessary, to reach the similar level of comparative research. Finally, the main subjects in our current study were patients who were pre-operatively assessed as having early stage esophageal cancer; therefore, theoretically no curative surgery was guaranteed for patients with positive lymph nodes. Since there were only a small number of lymph node-positive patients, it was impossible to judge whether the significantly lower survival rate in these patients were due to disease progression or incomplete lymph node dissection, which should be confirmed in further studies.
In conclusion, for patients with early stage esophageal cancer, the mediastinoscopic esophagectomy can achieve a similar effectiveness in curing this malignancy as the conventional thoracoscopic surgery through pleural cavity. Furthermore, the mediastinoscopic esophagectomy is featured by small surgical trauma and fast recovery in these patients.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Financial Disclosure: ZL Wang, XY Zhang.
References
- D’Journo XB, Thomas PA. Current management of esophageal cancer. J Thorac Dis 2014;6 Suppl 2:S253-64. [PubMed]
- Ishihara R, Yamamoto S, Hanaoka N, et al. Endoscopic submucosal dissection for superficial Barrett's esophageal cancer in the Japanese state and perspective. Ann Transl Med 2014;2:24. [PubMed]
- Ashtari S, Vahedi M. Economic burden of gastrointestinal cancer: estimation and importance. Transl Gastrointest Cancer 2014;3:178-81.
- Eblan MJ, Wang AZ. Improving chemoradiotherapy with nanoparticle therapeutics. Transl Cancer Res 2013;2:320-9. [PubMed]
- Lloyd S, Chang BW. Current strategies in chemoradiation for esophageal cancer. J Gastrointest Oncol 2014;5:156-65. [PubMed]
- Akl FM, Elsayed-Abd-Alkhalek S, Salah T. Palliative concurrent chemoradiotherapy in locally advanced and metastatic esophageal cancer patients with dysphagia. Ann Palliat Med 2013;2:118-23. [PubMed]
- Luketich JD, Pennathur A, Awais O, et al. Outcomes after minimally invasive esophagectomy: review of over 1000 patients. Ann Surg 2012;256:95-103. [PubMed]
- Luketich JD, Pennathur A, Franchetti Y, et al. Minimally invasive esophagectomy: results of a prospective phase II multicenter trial-the eastern cooperative oncology group (E2202) study. Ann Surg 2015;261:702-7. [PubMed]
- Biere SS, van Berge Henegouwen MI, Maas KW, et al. Minimally invasive versus open oesophagectomy for patients with oesophageal cancer: a multicentre, open-label, randomised controlled trial. Lancet 2012;379:1887-92. [PubMed]
- Koide N, Takeuchi D, Suzuki A, et al. Mediastinoscopy-assisted esophagectomy for esophageal cancer in patients with serious comorbidities. Surg Today 2012;42:127-34. [PubMed]
- Orringer MB, Marshall B, Iannettoni MD. Transhiatal esophagectomy: clinical experience and refinements. Ann Surg 1999;230:392-400; discussion 400-3. [PubMed]
- Wang QY, Tan LJ, Feng MX, et al. Video-assisted mediastinoscopic resection compared with video-assisted thoracoscopic surgery in patients with esophageal cancer. J Thorac Dis 2014;6:663-7. [PubMed]
- Feng MX, Wang H, Zhang Y, et al. Minimally invasive esophagectomy for esophageal squamous cell carcinoma: a case-control study of thoracoscope versus mediastinoscope assistance. Surg Endosc 2012;26:1573-8. [PubMed]
- Pop D, Venissac N, Mouroux J. Video-assisted mediastinoscopy improved radical resection for cancer in transhiatal esophagectomy. J Thorac Cardiovasc Surg 2007;133:267-8. [PubMed]
- Tangoku A, Yoshino S, Abe T, et al. Mediastinoscope-assisted transhiatal esophagectomy for esophageal cancer. Surg Endosc 2004;18:383-9. [PubMed]
- Wu B, Xue L, Qiu M, et al. Video-assisted mediastinoscopic transhiatal esophagectomy combined with laparoscopy for esophageal cancer. J Cardiothorac Surg 2010;5:132. [PubMed]
- Wang J, Jiang NQ, Jiang B, et al. Mediastinoscopy-assisted oesophagectomy in T1 oesophageal cancer patients with serious comorbidities: a 5-year long-term follow-up. Interact Cardiovasc Thorac Surg 2015;20:477-81. [PubMed]
- Binţinţan VV, Mehrabi A, Fonouni H, et al. Evaluation of the combined laparoscopic and mediastinoscopic esophagectomy technique. Chirurgia (Bucur) 2009;104:187-94. [PubMed]
- Liu J, Hu Y, Xie X, et al. Subcarinal node metastasis in thoracic esophageal squamous cell carcinoma. Ann Thorac Surg 2012;93:423-7. [PubMed]
- Ninomiya I, Okamoto K, Fujimura T, et al. Oncologic outcomes of thoracoscopic esophagectomy with extended lymph node dissection: 10-year experience from a single center. World J Surg 2014;38:120-30. [PubMed]