
What should we realistically expect from robotic bronchoscopy in the near future?
Robotic assisted bronchoscopy (RAB) platforms have several advantages over existing navigation technologies (1). The letter by Dr. Taichiro Goto entitled “Robotic Bronchoscopy: is it classic?” raises several important questions which we will address herein. Bronchoscopists expect that RAB will allow further reach into the peripheral airways while maintaining visualization, improve dexterity and safety of the peripheral nodule biopsy, improve diagnostic yield, and reduce procedure time and radiation exposure.
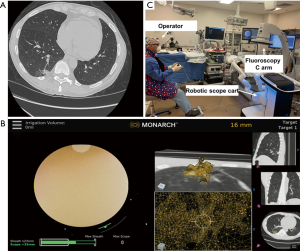
One RAB platform demonstrated further reach into the periphery as compared with a thin bronchoscope (9thvs. 6th generation) (2). The RAB platforms allows for improved dexterity due to the telescoping design and 4-way steering at the distal section. The improved visualization of the peripheral airway enables the bronchoscopist to advance and steer tools to overcome the narrow airways towards the target lesion. The ability to lock the scope into position allows for instruments to be advanced without exerting torque (2). Chen et al. showed successful localization in 96.2% (3). Chaddha et al. using the MonarchTM platform showed navigation success in 88.6% with a conservative and maximum diagnostic yield estimates of 69.1% and 77%, respectively (4). The exact diagnostic accuracy of this RAB platform for malignancy has not yet been established and we don’t know how it compares with other existing navigation technologies. In the NAVIGATE trial where electromagnetic navigation bronchoscopy was used for peripheral pulmonary lesion sampling, the diagnostic yield, sensitivity and negative predictive value at 12-month follow-up were 72.9%, 69% and 56%, respectively (5). Factors predicting diagnostic yield include the presence of a bronchus sign and lesion size ≥10 mm (3-5). The bronchus sign may not always be appreciated during pre-procedural planning since the airway is not always visualized due to the lack of contrast between the bronchus and surrounding emphysematous lung parenchyma. Recognizing pulmonary anatomy and knowing that vessels and airways are adjacent in the broncho-vascular bundle, we postulate that vessels can be used to help map a path towards the target lesion in patients without an obvious bronchus sign (Figure 1A,B). Time will tell whether the “vessel sign” results in a safe and efficient biopsy procedure.
A real concern with robotic technologies is the loss of tactile feedback. This is compensated by improvements in the reach, visualization, stability, and dexterity of the RAB platform (2,6,7). Future developments should continue to improve on peripheral visualization and further reduction in scope diameter and increased flexibility in order to augment scope maneuverability. Visualization is important during navigation and sampling of the lesions. Soiling of the lens during the procedure can pose a challenge to the operator. This can be mitigated by flushing air or saline, wiping the lens gently on the bronchial mucosa, or applying gentle suction in the distal airways while retracting the scope proximally (8).
Diagnostic yield may be improved in the near future if biopsies are performed during real-time imaging. The RAB platform has the advantage of locking the scope in position to allow for instruments to pass without moving the scope or exerting significant torque (2). Similar to EBUS-TBNA, however, the development of a scope or instrument that allows radial EBUS imaging and lung lesion sampling concurrently can minimize the need to switch instruments and could improve yield.
For the foreseeable future, RAB will remain an artificial intelligence tool but not a self-driving system that employs a collision avoidance system. Manual but guided navigation is critical, especially when accessing the distal airways where the airway anatomy may not always be clear on CT imaging. Additionally, even though airway trauma should be avoided, contact with the airway is sometimes relied upon in order to manipulate the bronchoscope across the bends of distal carinas and transverse into the distal airways. This maneuver of gently advancing the scope in the peripheral airways without tactile feedback may be concerning as it could theoretically lead to significant airway trauma followed by pneumothorax or bleeding. That being said, published human trials have demonstrated overall safety of a RAB platform, with complication rates comparable to conventional bronchoscopy (3,4). There are no published reports of airway bleeding that have required the use of blood transfusion, open thoracotomy, or the use of endobronchial blockers (3,4). This may be related to the relatively low-pressure vascular system in the distal lung. However, bleeding may become more of a concern as this technology continues to develop and potentially be used for bronchoscopic therapeutic ablation of inoperable malignant lesions. Our current approach is to wedge the sheath of the scope in a segmental or sub-segmental airway and then advance the scope; this will avoid spilling of blood into the normal lung. In case of bleeding, we apply cold saline followed by continuous suctioning in the wedged position which will collapse the distal airway. While it is easy to disconnect, remove the robotic bronchoscope and introduce a therapeutic flexible bronchoscope, the act of switching scopes does have the potential risk of losing a wedged position, anatomical orientation and potentially worsening the consequences of an otherwise localized and isolated bleed.
It remains unknown whether RAB reduces radiation exposure to patients and operators. Reducing the time of fluoroscopy is desirable with any guided bronchoscopy. However, the RAB offers a potential advantage. The sources of radiation exposure to staff include scattered radiation from the patient and the X-ray tube leakage. To reduce radiation exposure, it is advisable to increase the operator-patient distance during fluoroscopy. For instance, by standing at 2 meters instead of 0.5 meters from the center of the table, the operators will reduce their exposure by a factor of 16 (9). This is possible during RAB (Figure 1C).
Many of these questions will be answered by the ongoing large, prospective, multi-centered TARGET study (ClinicalTrials.gov Identifier: NCT04182815) which is evaluating the incidence of device and procedure-related complications up to 7-days post-procedure, the total procedure time, rate of conversion to alternative procedures and diagnostic yield and accuracy for malignancy. Ultimately, comparative trials of existing guided bronchoscopy platforms will be needed to evaluate the cost-effectiveness of these technologies in sampling and diagnosing peripheral pulmonary lesions.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Journal of Thoracic Disease. The article did not undergo external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd-20-3323). Dr. DKH reports personal fees from Olympus/Spiration, personal fees from PulmonX, during the conduct of the study; personal fees and other from Auris, personal fees from Ambu, personal fees, non-financial support and other from Body Vision, personal fees and other from Eolo, other from Eon, other from Gravitas, personal fees and other from Noah Medical, personal fees and other from LX-Medical, other from Med-Opsys, other from Monogram Orthopedics, personal fees and other from Preora, other from VIDA, other from Viomics, grants and personal fees from Boston Scientific, personal fees from Johnson and Johnson, personal fees from oncocyte, personal fees from veracyte, personal fees and other from Broncus, grants and personal fees from Gala, personal fees from Heritage Biologics, personal fees from IDbyDNA, personal fees from Level-Ex, personal fees from Medtronic, personal fees from Neurotronic, personal fees from olympus, personal fees from PulmonX, personal fees from Astra-Zeneca, personal fees from Biodesix, personal fees from Genetech, personal fees from Grifols, personal fees from Takeda, personal fees from CSL, personal fees from InhibRX, personal fees and other from Prothea-X, outside the submitted work. Dr. SM reports other from Olympus, other from Boston Scientific, grants from Pinnacle Biologics, other from Cook Medical, other from Johnson and Johnson, grants from Medtronic, outside the submitted work. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Agrawal A, Hogarth DK, Murgu S. Robotic bronchoscopy for pulmonary lesions: a review of existing technologies and clinical data. J Thorac Dis 2020;12:3279-86. [Crossref] [PubMed]
- Chen AC, Gillespie CT. Robotic Endoscopic Airway Challenge: REACH Assessment. Ann Thorac Surg 2018;106:293-7. [Crossref] [PubMed]
- Chen AC, Pastis NJ Jr, Mahajan AK, et al. Robotic Bronchoscopy for Peripheral Pulmonary Lesions: A Multicenter Pilot and Feasibility Study. Chest 2020. Epub ahead of print. [Crossref] [PubMed]
- Chaddha U, Kovacs SP, Manley C, et al. Robot-assisted bronchoscopy for pulmonary lesion diagnosis: results from the initial multicenter experience. BMC Pulm Med 2019;19:243. [Crossref] [PubMed]
- Folch EE, Pritchett MA, Nead MA, et al. Electromagnetic Navigation Bronchoscopy for Peripheral Pulmonary Lesions: One-Year Results of the Prospective, Multicenter NAVIGATE Study. J Thorac Oncol 2019;14:445-58. [Crossref] [PubMed]
- Chen AC, Pastis NJ, Machuzak MS, et al. Accuracy of a Robotic Endoscopic System in Cadaver Models with Simulated Tumor Targets: ACCESS Study. Respiration 2020;99:56-61. [Crossref] [PubMed]
- Yarmus L, Akulian J, Wahidi M, et al. A Prospective Randomized Comparative Study of Three Guided Bronchoscopic Approaches for Investigating Pulmonary Nodules: The PRECISION-1 Study. Chest 2020;157:694-701. [Crossref] [PubMed]
- Murgu SD. Robotic assisted-bronchoscopy: technical tips and lessons learned from the initial experience with sampling peripheral lung lesions. BMC Pulm Med 2019;19:89. [Crossref] [PubMed]
- Schueler BA, Vrieze TJ, Bjarnason H, et al. An investigation of operator exposure in interventional radiology. Radiographics 2006;26:1533-41. [Crossref] [PubMed]


