
Elevated plasma Sirtuin2 level predicts heart failure after acute myocardial infarction
Introduction
Sirtuin (SIRT) is a family of NAD+-dependent histone deacetylases, regulating metabolism and aging-related diseases (e.g., diabetes, cancer, neurodegenerative and cardiovascular diseases) (1-5). Recently, SIRT2, as a member of sirtuin family, was reported to play a significant role in cardiovascular disease. It has been previously shown that over expression of cardiac-specific SIRT2, promoting AMP-activated protein kinase (AMPK) activation, can protect heart against Ang II-induced cardiac hypertrophy and fibrosis (6), while SIRT2 can repress nuclear factor of activated T-cells (NFAT) to maintain cardiac homeostasis and ameliorate cardiac dysfunction (7). In addition, SIRT2 gene is down-regulated in the cardiac tissue in cardiosurgical patients undergoing remote ischemic preconditioning (8), and functional genetic variants in acute myocardial infarction(AMI) patients were observed as well (9).
It is noteworthy that, SIRT2 is expressed in various metabolically relevant tissues (e.g., the heart, brain, and adipose tissue) (10), and is also detected in circulation (11). SIRT2 plays a pivotal role in various physiological processes in maintaining metabolic homeostasis, including inflammation, oxidative stress, and mitochondrial function, as well as adipocyte differentiation, fatty acid oxidation, and insulin sensitivity. SIRT2 may enhance acetylation and activation of NF-κB p65 (12,13) and regulate expression of CXCL2 and CCL2 (14) to suppress inflammatory process, while regulate acetylation of G6PD to modulate NADPH homeostasis and cell survival during oxidative stress (15). Dysregulated SIRT2 activity has been found to be associated with inflammatory and metabolic disorders (10,16). Moreover, AMI, leading to the highest mortality among cardiovascular diseases, is involved in both metabolic dysfunction and inflammatory responses (17-19). The cause of AMI patients’ death is either heart failure or a malignant arrhythmia, especially heart failure, which is closely associated with inflammatory responses and contributes to long-term mortality after AMI (20,21).
However, no study has concentrated on the role of circulating SIRT2 in AMI yet. In the present study, we investigated the relationship between plasma SIRT2 level and AMI, and evaluated the association of plasma SIRT2 level with major adverse cardiovascular events (MACE) and heart failure after AMI. Our results clarified the role of plasma SIRT2 level in AMI prognosis. We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/jtd-20-2234).
Methods
Study subjects
This is a prospective observational study. Study subjects were consecutively recruited from Beijing Chao-yang Hospital (Beijing, China) between October 2018 to March 2019. A total of 129 AMI patients [including 74 ST-segment elevation myocardial infarction (STEMI) and 55 non-ST-segment elevation myocardial infarction (NSTEMI)] with heart attack within 12 hours were enrolled in the present study. All patients successfully underwent revascularization in emergency before hospitalization. The diagnosis of AMI was carried out at the time of admission on the basis of criteria, including clinical symptoms, typical changes in electrocardiogram (ECG), elevated cardiac biomarkers (cardiac troponin-I and creatine kinase MB). The exclusion criteria were as follows: neoplasm, severe organ failure, or other infectious or inflammatory conditions. Written informed consent was obtained from all the participants. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) (22), and the research protocol was approved by the Ethics Committee of Beijing Chao-Yang Hospital (No. 2018-2-7-3) and informed consent was taken from all the patients.
Clinical conditions
For all the AMI patients data related to cardiac arrest, utilization of intra-aortic balloon pump (IABP), and breathing machine, and death during hospitalization were recorded, and all the patients were followed-up for 12 months. The MACE included cardiac death, readmission for revascularization and heart failure. Heart failure involved death due to heart failure during hospitalization, and readmission because of heart failure after discharge.
Laboratory measurements
Baseline laboratory measurements were obtained within the first 12 hours of admission. Plasma of SIRT2 levels were assayed using a commercially available enzyme-linked immunosorbent assay (ELISA) kit (R&D Systems Inc., Minneapolis, MN, USA) according to the manufacturer’s instructions. The coefficient of variation for the assay was <5%. Fasting venous blood samples were collected to measure the levels of glucose, homocysteine, creatinine, and lipids (including the levels of total cholesterol, high-density lipoprotein cholesterol, and triglycerides).
Statistical analysis
Continuous variables were expressed as mean ± standard deviation (SD) and quartiles, while categorical variables were expressed as percentages and numbers. Comparisons between groups were performed using chi-square test for categorical variables, and two-sample t-test for comparing continuous variables in normally distributed status, as well as Kruskal-Wallis test for comparing continuous variables in non-normally distributed status. Pearson’s and Spearman’s correlation coefficients were used for comparing parametric and nonparametric variables, respectively. Cox proportional hazards analysis was carried out to determine the independent predictors of MACE. All the analyses were performed using SPSS 24.0 software (IBM, Armonk, NY, USA), and a 2-tailed P<0.05 was considered statistically significant.
Results
Study subjects’ clinical characteristics
A total of 129 AMI patients (mean age: 62.2±12.7 years old, male/female: 96/33) were enrolled in the present study, including 74 STEMI and 55 NSTEMI. The median follow-up period was 8 months, and during the follow-up, 22 (17.1%) and 16 (12.4%) AMI patients experienced MACE and heart failure respectively. The median value (25th, 75th percentiles) of plasma SIRT2 level was 69.0 (48.9, 109.0) pg/mL. According to the 75th percentile value of plasma SIRT2 level, we divided all the patients into high-level group (plasma SIRT2 level ≥109.0 pg/mL) and low-level group (plasma SIRT2 level <109.0 pg/mL), and clinical parameters between the two groups were compared (Table 1) . Compared with the low-level group, the high-level group had significantly higher levels of C-reactive protein (CRP), blood urea nitrogen (BUN), and serum creatinine.
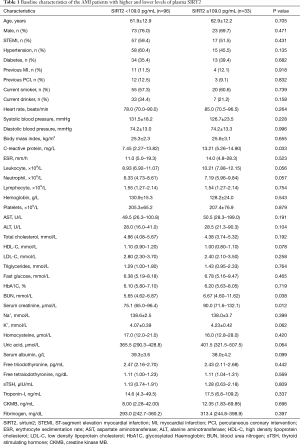
Full table
Association between plasma SIRT2 level with indicators of AMI severity
The indicators of AMI severity included MACE, mortality, heart failure, utilization of IABP and breathing machine, malignant arrhythmia, cardiac arrest, Killip class, left ventricular ejection fraction (LVEF), plasma brain natriuretic peptide (BNP) level, treatment, occlusive lesions, collateral circulation, as well as SYNTAX scores and GRACE scores. Compared with the low-level group (plasma SIRT2 level <109.0 pg/mL), the high-level group (plasma SIRT2 level ≥109.0 pg/mL) was found to have higher percentage of MACE (P<0.001), heart failure (P<0.001), breathing machine use (P=0.003), Killip class ≥3 (P<0.001), LVEF <50% (P=0.007) or even <40% (P=0.012), and higher BNP level (P=0.006) (Table 2).
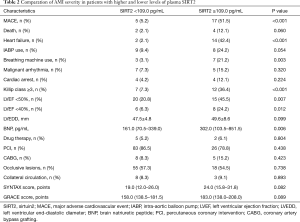
Full table
Relationships between plasma SIRT2 level and clinical parameters
Plasma SIRT2 level was noted to be associated with leukocyte and neutrophil (r=0.209, P=0.018 for leukocyte; r=0.217, P=0.014 for neutrophil), and also correlated with erythrocyte sedimentation rate (ESR) and CRP (r=0.215, P=0.025 for ESR; r=0.265, P=0.004 for CRP). Additionally, plasma SIRT2 level was also correlated with renal function (r=0.183, P=0.039 for serum creatinine; r=0.279, P=0.001 for renal dysfunction) and heart rate (r=0.199, P=0.024). However, plasma SIRT2 level was not associated with patient’s age, gender, the levels of blood glucose and lipid, cardiac Troponin-I, creatine kinase MB (CKMB), as well as GRACE and SYNTAX scores (Table 3).
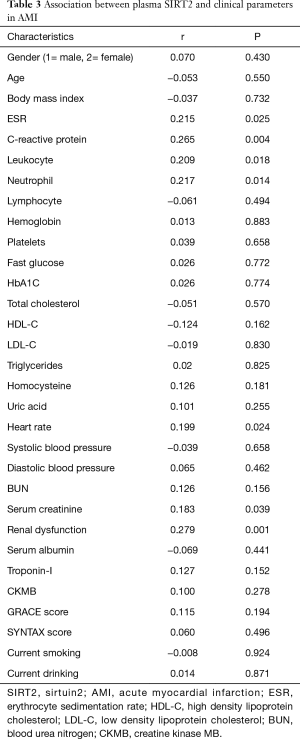
Full table
Univariate and multivariate Cox analysis of predictors of MACE and heart failure
A total of 22 AMI patients had MACE recorded (including 20 cases of readmission and 16 cases of heart failure), that 2 patients died of malignant arrhythmia during hospitalization, 4 patients died of heart failure during readmission, 12 patients recovered from heart failure during readmission, and 4 patients underwent revascularization during readmission. Univariate Cox regression analysis showed that elder, renal dysfunction, higher plasma SIRT2 level, greater SYNTAX and GRACE scores, malignant arrhythmia, cardiac arrest, Killip class ≥3, LVEF <50% or <40%, BNP >500 ng/L, in addition to application of IABP and breathing machine were associated with higher risks of MACE and heart failure. The GRACE scores were calculated using patient’s age, heart rate, systolic blood pressure, creatinine, Killip class, ST-segment deviation, elevated cardiac enzyme level, and cardiac arrest at the time of admission; for this purpose, we included GRACE and SYNTAX scores, gender, body mass index (BMI), diabetes, plasma SIRT2 level, BNP >500 ng/L, as well as utilization of IABP and breathing machine in the multivariate Cox regression analysis. Higher plasma SIRT2 level and GRACE score were associated with higher risk of MACE [for plasma SIRT2 level: hazard ratio (HR) 11.20, 95% confidence interval (CI): 3.18–39.52, P<0.001; for GRACE score: HR 1.03, 95% CI: 1.02–1.05, P<0.001] and heart failure (for plasma SIRT2 level: HR 27.10, 95% CI: 4.65–157.83, P<0.001; for GRACE score: HR 1.03, 95% CI: 1.01–1.05, P=0.009), while use of breathing machine was associated with higher risk of MACE (HR 12.16, 95% CI: 2.37–62.26, P=0.003) and heart failure (HR 11.45, 95% CI: 1.80–72.97, P=0.010) (Table 4, Figure 1).
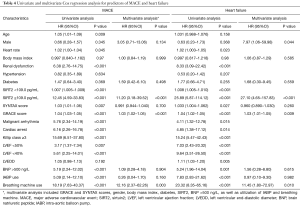
Full table
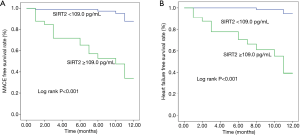
Discussion
In the present study, we, for the first time, assessed the role of plasma SIRT2 level in AMI patients. We found that plasma SIRT2 was an appropriate biomarker to predict heart failure and MACE after AMI. The results showed that, compared with AMI patients with lower plasma SIRT2 level, those cases with higher plasma SIRT2 level had worse cardiac function and higher risk of MACE during hospitalization and in the follow-up after discharge.
Accumulating evidence has uncovered SIRT2 played important roles in human diseases. SIRT2 showed increased levels in plasma in patients with cervical cancer compared with controls, and was considered as a potential biomarker to diagnose cervical cancer (11). SIRT2 was also increased in the peripheral blood from Alzheimer’s disease (AD) subjects and elderly controls compared to levels in healthy young control, and might possibly be considered peripheral markers of AD (23). Circulating SIRT2 and other inflammatory biomarkers were significantly higher in rheumatoid arthritis (RA) patients with periodontal disease (PD) than RA without PD, indicating a augmented systemic inflammation status (24).
Previous studies demonstrated that SIRT2 was a protective factor in cardiovascular disease and also showed that expression levels of SIRT2 protein were down-regulated in cardiomyocytes treated with phenylephrine or isoproterenol (7), as well as in hypertrophic hearts of mice (6) or even in hearts of T1DM rats (25). Sirt2-KO markedly exaggerated cardiac hypertrophy and fibrosis, as well as causing decreases of cardiac ejection fraction and fractional shortening in aged mice and Ang II-infused mice (6); besides, overexpression of SIRT2 attenuated agonist-induced cardiac hypertrophy in cardiomyocytes (7). Moreover, SIRT2 mediated hypertension-induced vascular remodeling (26). In cardiosurgical patients undergoing remote ischemic preconditioning, SIRT2 gene was down-regulated in the cardiac tissue (8). Functional genetic variants within the SIRT2 gene promoter were found in AMI patients (9). It was previously reported that SIRT2 played a substantial role in cardiovascular disease. In addition, SIRT2 has been detected in the circulation (11), however, there is no evidence about the role of circulating SIRT2 in AMI. In the present study, we noted that circulating SIRT2 was an acceptable biomarker for AMI, and the higher plasma SIRT2 level was associated with poorer AMI prognosis, especially for worse cardiac function.
In the present study, plasma SIRT2 level was found to be correlated with counts of leukocytes and neutrophils, as well as plasma levels of ESR and CRP, which were consistent with the results of previously conducted studies. Moreover, the results of present research unveiled that plasma SIRT2 level was mainly correlated with the indicators of heart failure after AMI, while that wasn’t correlated with myocardial enzyme or severity of coronary artery stenosis (evaluated by SYNTAX score). We also found that in AMI patients, higher plasma SIRT2 level was associated with worse cardiac function. Inflammatory response is a key risk factor for heart failure after AMI (27). Overexpression of SIRT2 suppressed inflammatory responses and reactive oxygen species–induced macrophage cytotoxicity (28-30). Inflammatory responses in AMI patients were also associated with malignant arrhythmia (31,32), and higher plasma SIRT2 level was noted to have higher percentage of malignant arrhythmia and cardiac arrest in the present study.
Additionally, SIRT2 was reported to be associated with renal inflammatory injury, in which SIRT2 showed an anti-inflammatory effect through regulating p65 binding to the promoters of CXCL2 and CCL2 (14). In the current study, plasma SIRT2 level was correlated with renal function in AMI, which might be due to the inflammatory responses in AMI patients.
The present study contains a number of limitations: (I) the period of follow-up was short, therefore, long-term follow-up studies need to be conducted to further analyze the association between plasma SIRT2 level and AMI; (II) this is a study with small sample size; large sample size should be carried out to verify our findings.
Conclusions
Our findings revealed that plasma SIRT2 level was a proper biomarker to predict heart failure and MACE after AMI, which, as indicated by previous studies, might be through regulating metabolic and inflammatory pathways.
Acknowledgments
Funding: This research was supported by Natural Science Foundation of China (No. 81800304).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/jtd-20-2234
Data Sharing Statement: Available at http://dx.doi.org/10.21037/jtd-20-2234
Peer Review File: Available at http://dx.doi.org/10.21037/jtd-20-2234
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd-20-2234). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of Beijing Chao-Yang Hospital (No. 2018-2-7-3) and informed consent was taken from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bonkowski MS, Sinclair DA. Slowing ageing by design: the rise of NAD(+) and sirtuin-activating compounds. Nat Rev Mol Cell Biol 2016;17:679-90. [Crossref] [PubMed]
- Winnik S, Auwerx J, Sinclair DA, et al. Protective effects of sirtuins in cardiovascular diseases: from bench to bedside. Eur Heart J 2015;36:3404-12. [Crossref] [PubMed]
- Yamamoto H, Schoonjans K, Auwerx J. Sirtuin functions in health and disease. Mol Endocrinol 2007;21:1745-55. [Crossref] [PubMed]
- Donmez G, Outeiro TF. SIRT1 and SIRT2: emerging targets in neurodegeneration. EMBO Mol Med 2013;5:344-52. [Crossref] [PubMed]
- Matsushima S, Sadoshima J. The role of sirtuins in cardiac disease. Am J Physiol Heart Circ Physiol 2015;309:H1375-89. [Crossref] [PubMed]
- Tang X, Chen XF, Wang NY, et al. SIRT2 Acts as a Cardioprotective Deacetylase in Pathological Cardiac Hypertrophy. Circulation 2017;136:2051-67. [Crossref] [PubMed]
- Sarikhani M, Maity S, Mishra S, et al. SIRT2 deacetylase represses NFAT transcription factor to maintain cardiac homeostasis. J Biol Chem 2018;293:5281-94. [Crossref] [PubMed]
- Zitta K, Meybohm P, Gruenewald M, et al. Profiling of cell stress protein expression in cardiac tissue of cardiosurgical patients undergoing remote ischemic preconditioning: implications for thioredoxin in cardioprotection. J Transl Med 2015;13:34. [Crossref] [PubMed]
- Yang W, Gao F, Zhang P, et al. Functional genetic variants within the SIRT2 gene promoter in acute myocardial infarction. PLoS One 2017;12:e0176245. [Crossref] [PubMed]
- Gomes P, Fleming Outeiro T, Cavadas C. Emerging Role of Sirtuin 2 in the Regulation of Mammalian Metabolism. Trends Pharmacol Sci 2015;36:756-68. [Crossref] [PubMed]
- Berggrund M, Enroth S, Lundberg M, et al. Identification of Candidate Plasma Protein Biomarkers for Cervical Cancer Using the Multiplex Proximity Extension Assay. Mol Cell Proteomics 2019;18:735-43. [Crossref]
- Yuan F, Xu ZM, Lu LY, et al. SIRT2 inhibition exacerbates neuroinflammation and blood-brain barrier disruption in experimental traumatic brain injury by enhancing NF-kappaB p65 acetylation and activation. J Neurochem 2016;136:581-93. [Crossref] [PubMed]
- Rothgiesser KM, Erener S, Waibel S, et al. SIRT2 regulates NF-kappaB dependent gene expression through deacetylation of p65 Lys310. J Cell Sci 2010;123:4251-8. [Crossref] [PubMed]
- Jung YJ, Lee AS, Nguyen-Thanh T, et al. SIRT2 Regulates LPS-Induced Renal Tubular CXCL2 and CCL2 Expression. J Am Soc Nephrol 2015;26:1549-60. [Crossref] [PubMed]
- Wang YP, Zhou LS, Zhao YZ, et al. Regulation of G6PD acetylation by SIRT2 and KAT9 modulates NADPH homeostasis and cell survival during oxidative stress. EMBO J 2014;33:1304-20. [Crossref] [PubMed]
- German NJ, Haigis MC. Sirtuins and the Metabolic Hurdles in Cancer. Curr Biol 2015;25:R569-83. [Crossref] [PubMed]
- Weil BR, Neelamegham S. Selectins and Immune Cells in Acute Myocardial Infarction and Post-infarction Ventricular Remodeling: Pathophysiology and Novel Treatments. Front Immunol 2019;10:300. [Crossref] [PubMed]
- Lim GB. Acute coronary syndromes: Supplemental oxygen in myocardial infarction. Nat Rev Cardiol 2017;14:632. [PubMed]
- Synetos A, Papanikolaou A, Toutouzas K, et al. Metabolic syndrome predicts plaque rupture in patients with acute myocardial infarction. An optical coherence study. Int J Cardiol 2016;209:139-41. [Crossref] [PubMed]
- Ye F, Winchester D, Jansen M, et al. Assessing Prognosis of Acute Coronary Syndrome in Recent Clinical Trials: A Systematic Review. Clin Med Res 2019;17:11-9. [Crossref] [PubMed]
- Davis WT, Montrief T, Koyfman A, et al. Dysrhythmias and heart failure complicating acute myocardial infarction: An emergency medicine review. Am J Emerg Med 2019;37:1554-61. [Crossref] [PubMed]
- World Medical A. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA 2013;310:2191-4. [Crossref] [PubMed]
- Wongchitrat P, Pakpian N, Kitidee K, et al. Alterations in the Expression of Amyloid Precursor Protein Cleaving Enzymes mRNA in Alzheimer Peripheral Blood. Curr Alzheimer Res 2019;16:29-38. [Crossref] [PubMed]
- Panezai J, Ali A, Ghaffar A, et al. Upregulation of circulating inflammatory biomarkers under the influence of periodontal disease in rheumatoid arthritis patients. Cytokine 2020;131:155117. [Crossref] [PubMed]
- Yuan Q, Zhan L, Zhou QY, et al. SIRT2 regulates microtubule stabilization in diabetic cardiomyopathy. Eur J Pharmacol 2015;764:554-61. [Crossref] [PubMed]
- Hashimoto-Komatsu A, Hirase T, Asaka M, et al. Angiotensin II induces microtubule reorganization mediated by a deacetylase SIRT2 in endothelial cells. Hypertens Res 2011;34:949-56. [Crossref] [PubMed]
- Nahrendorf M, Frantz S, Swirski FK, et al. Imaging systemic inflammatory networks in ischemic heart disease. J Am Coll Cardiol 2015;65:1583-91. [Crossref] [PubMed]
- Kim MJ, Kim DW, Park JH, et al. PEP-1-SIRT2 inhibits inflammatory response and oxidative stress-induced cell death via expression of antioxidant enzymes in murine macrophages. Free Radic Biol Med 2013;63:432-45. [Crossref] [PubMed]
- Eskandarian HA, Impens F, Nahori MA, et al. A role for SIRT2-dependent histone H3K18 deacetylation in bacterial infection. Science 2013;341:1238858. [Crossref] [PubMed]
- Pais TF, Szego EM, Marques O, et al. The NAD-dependent deacetylase sirtuin 2 is a suppressor of microglial activation and brain inflammation. EMBO J 2013;32:2603-16. [Crossref] [PubMed]
- Kobayashi Y. How to manage various arrhythmias and sudden cardiac death in the cardiovascular intensive care. J Intensive Care 2018;6:23. [Crossref] [PubMed]
- Frangogiannis NG. The inflammatory response in myocardial injury, repair, and remodelling. Nat Rev Cardiol 2014;11:255-65. [Crossref] [PubMed]


