
Clock dial integrated positioning combined with single utility port video-assisted thoracoscopic surgery: a new localization method for lung tumors
Introduction
With advances in the global economy and, the number of people with lung tumors has dramatically increased worldwide (1,2). An increasing number of patients with lung tumors and ground glass nodules (GGNs) have been identified through computed tomography (CT), with the majority of these cases being early-stage lung cancers. Video-assisted thoracoscopic surgery (VATS) with systemic lymph node dissection is considered as the standard treatment for early non-small cell lung cancer (NSCLC) (3,4). Nowadays, preoperative invasive localization of lung tumor mainly consists of two methods: CT-guided localization (5-9) and electromagnetic navigation bronchoscopy (ENB)-guided localization (10-12). However, these methods have some disadvantages such as the lack of conditions in some backward areas, increased preoperative trauma and anxiety, etc. As well as some serious complications such as intrapulmonary focal hemorrhage, pneumothorax, chest pain requiring analgesia, hemosputum and so on (5-7).
The purpose of this study was to introduce a new intraoperative noninvasive localization method to locate lung tumors, which showed satisfactory results. This is the first report about the application of clock dial integrated positioning (CDIP) combined with single utility port VATS (SUPVATS) in the treatment of lung tumors. We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/jtd-20-3312).
Methods
Patient selection
Between June 2017 and October 2017, a total of 127 patients with lung tumors underwent CDIP combined with SUPVATS at the Shanghai Chest Hospital of Shanghai Jiao Tong University. The patients’ characteristics are listed in Table 1. Clinical staging of NSCLC was based on the Union for International Cancer Control (UICC) staging (8th edition) (13). All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Institutional Review Board and Ethics Committee of the Shanghai Chest Hospital of Shanghai Jiao Tong University. All patients underwent preoperative routine echocardiography, abdominal ultrasonography, pulmonary function measurement, blood examination, chest CT scan, bone scintigraphy scan, and brain magnetic resonance imaging (MRI) to exclude extra-thoracic spread of the disease. The detailed medical history of all patients was documented, and all patients granted their informed consent at least one day before surgery, following conscientious explanation of the procedure and the goals of the study.
Full table
The inclusion criteria (14) were as follows: (I) initial stages of NSCLC T1N0M0 to T1N1M0, or isolated single N2 lymphadenectasis of phase IIIA; (II) for smokers, the patient ceased smoking for at least 2 weeks prior to the operation; (III) preoperative lung function: forced expiratory volume (FEV1)% and maximal voluntary ventilation (MVV)% of predicted >50%, FEV1 >1 L; and (IV) no history of thoracic surgery or severe chest wall deformity. The exclusion criteria (14) were as follows: (I) tumor invasion of the large vessels of the mediastinum or important nerves such as the recurrent laryngeal nerve; (II) tumor invasion of large chest walls requiring reconstruction; (III) multi-station lymphatic metastasis; (IV) tumor invasion of the protuberance or trachea; (V) mediastinal lymphoid tuberculosis; (VI) severe chest wall deformity; (VII) upper airway and maxillofacial injury or deformity; and (VIII) lung, pleural, or heart disease.
Surgical procedure
Localization of lung tumors using CDIP
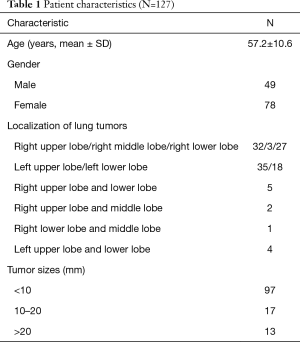
The CDIP method was applied to achieve intraoperative localization, based on the preoperative CT scans. The right chest with the Azygos vein arch and lower pulmonary vein, and the left chest with the aortic arch and the lower pulmonary vein, were tagged, and the lung was divided into the upper, middle, and lower parts according to the anatomical structure of the chest (Figure 1). On the right side of the tumors, the CT was rotated 90 degrees clockwise (Figure 2A,B). On the left side of the tumors, the CT was rotated 90 degrees counterclockwise (Figure 2C,D). CDIP was used to locate the midaxillary line at the 12 o’clock position, and the upper, middle, and lower parts of the lung were preoperatively identified to accurately locate the tumors. The vertical distance and clock dial points were used to locate the lung tumor.

The specific measures were as follows: firstly, based on the CT scan, we calculated the vertical distance of the tumors (according to the azygos vein arch, aortic arch, and lower pulmonary vein), and determined the part in which the tumor was located (Figure 1). Secondly, we calculated the clock dial position of the tumors according to the above method (Figure 2). Thirdly, we used an electrotome to mark the tumor site in the lung. Lastly, the operations were centered in that region, and thoracoscopic surgery was performed.
Surgical technique and localization procedure
Under general anesthesia with a double-lumen tube in the lateral position, the thoracoscopy monitors were placed on the back of the patient, and both the surgeon and the assistant stood on the ventral side of the patient. According to the location of the tumors, SUPVATS lung resection was performed with a 2-cm working port and a 1-cm observation port. The observation port was placed at the 7th intercostal space (ICS) on the middle axillary line, and thoracoscope with 30° was inserted. The working (utility) port was placed at the 3rd or 4th ICS on the anterior axillary line.
We calculated the exact location of the tumors based on the preoperative CT scan (Figure 3). If the tumor was difficult to locate, the anesthesiologist would drum the lung so that we could accurately determine the location of the tumor in the expanded state. SUPVATS excision of the tumors included lobectomy, segmentectomy, wedge resection, and mediastinal lymph node dissection or sampling. If 13th or 11th lymph node metastasis was confirmed, we extended the resection or performed lobectomy, as required. In general, most tumors were resected in the first thoracoscopic surgery, while other tumors were resected by extended resection or lobectomy. When pleural irrigation and lung expansion showed no active hemorrhage or air leakage, a chest tube was placed directly through the observation port (14). The chest tube was removed (14,15) when: (I) the amount of drainage was <100 mL/day; (II) the lung was fully expanded without pleural effusion on chest radiography; and (III) there was no air leakage through the chest tube.

Data collection and follow-up
The surgical type, operation time, intraoperative bleeding, postoperative hospital stay, chest drain duration, postoperative pathological TNM stage of NSCLC, and complications were recorded. Among the 127 patients, 116 cases (91.34%) were followed-up, while 11 cases (8.66%) were lost to follow-up.
Statistical analysis
Clinicopathologic data was analyzed using the SPSS 22.0 software package (SPSS Inc., Chicago, IL). Continuous variables were expressed as mean ± standard deviation (SD). Categoric variables were expressed as count and percentages.
Results
Surgical methods and related data
In this study, only 3 cases (2.36%) underwent thoracoscopic biopsy due to incomplete resection, while the remaining 124 cases (97.64%) underwent thoracoscopic surgery. This included 14 cases of lobectomy, 107 cases of partial resection (including segmental or wedge resection), 2 cases of lobectomy and partial resection, 1 case of left total pneumonectomy, and 3 cases of thoracoscopic biopsy. In two patients, the lung tumors could not be found during the first thoracoscopic surgery, but were subsequently removed by lobectomy and extended resection, respectively.
General postoperative and pathology data
The mean operation time and intraoperative bleeding were 47.9±22.1 min and 70.1±40.3 mL, respectively. The mean postoperative hospital stay and chest drain duration were 3.9±2.2 and 3.6±1.8 days, respectively. There were 118 cases of malignant tumors, including 101 cases of adenocarcinoma, 9 cases of squamous cell carcinoma, 2 cases of large cell carcinoma, 3 cases of small cell lung carcinoma, and 3 cases of metastatic lung carcinoma. The remaining 9 cases were benign tumors, including 3 cases of granuloma, 2 cases of intrapulmonary lymph node, 2 cases of sclerosing hemangioma, and 2 cases of hamartoma. According to the clinical staging of NSCLC based on the UICC staging (8th edition) (13), 83 cases were stage IA1, 10 cases were stage IA2, 7 cases were stage IA3, 2 cases were stage IB, 2 cases were stage IIA, 2 cases were stage IIB, and 6 cases were stage IIIA. The clinical characteristics of the 127 patients are shown in Table 2.
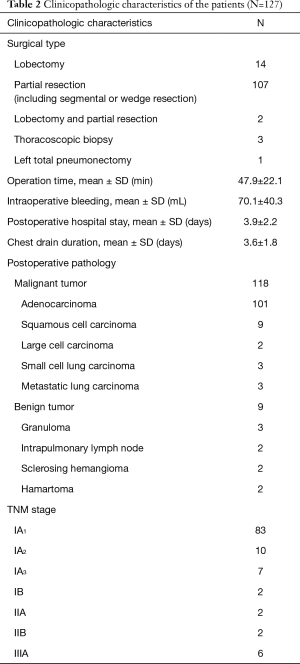
Full table
Data of postoperative complications and follow-up
All patients were hospitalized. The incidence of postoperative complications was 10.2%; postoperative pneumonia was observed in 7 cases (5.51%), atelectasis in 2 cases (1.57%), prolonged air leakage (>5 days) in 2 cases (1.57%), hypoxia in 1 case (0.79%), and arrhythmia in 1 case (0.79%). The postoperative complications are shown in Table 3. All patients were successfully discharged after surgery, and there was no mortality, secondary operation, or conversion to open procedure due to massive intraoperative bleeding. The 116 patients were followed-up for 6-12 months by outpatient (103 cases, 88.79%), telephone (10 cases, 8.62%), and e-mail (3 cases, 2.59%), respectively. The mean duration of follow-up was 9.1±1.8 months. Two cases developed pulmonary metastasis at 6 months and bone metastases at 11 months after surgery, respectively. No deaths occurred during the follow-up period.

Full table
Discussion
The department of thoracic surgery at the Shanghai Chest Hospital of Shanghai Jiao Tong University is the largest thoracic centre in China, and deals with the complete spectrum of diseases and disorders. We have performed more than 10,000 thoracic surgeries in 2019; thoracoscopic surgery accounted for >80% of cases, and robotic surgery accounted for >3% of cases. There are numerous early-stage lung cancer patients at our centre, and the localization of lung tumors is a primary concern. Various methods are used in our clinic for the localization of lung tumors, including the hook wire, vital dye, transbronchial, and CDIP. At present, local resection has become an option for early-stage lung cancer, which requires us to be able to pinpoint the location of the lung tumor, so that we can ensure cutting margin for surgery in order to preserve more normal tissue.
In this study, only 3 cases (2.36%) underwent thoracoscopic biopsy due to incomplete resection, while the remaining 124 cases (97.64%) underwent thoracoscopic surgery. In two patients, the lung tumors could not be found during the first thoracoscopic surgery, but were subsequently removed by lobectomy and extended resection, respectively. Tables 2 and 3 demonstrate satisfactory efficacy and low postoperative complications in all patients. All patients were successfully discharged after surgery, and there was no mortality, secondary operation, or conversion to open procedure due to massive intraoperative bleeding. During the follow-up (after stenting), two cases developed pulmonary metastasis at 6 months and bone metastases at 11 months after surgery, respectively. No deaths occurred during the follow-up period.
The ability of our team to achieve these positive outcomes is attributable to several factors. Firstly, Professor Li has extensive surgical experience, and has performed several thousand thoracic surgeries. A previous study (16) showed that a low complication rate was achieved if the surgery was performed by experienced surgeons after proper training. Secondly, we have implemented complete perioperative education and guidance for patients and their families. We urge patients to give up smoking, strengthen nutritional support, improve perioperative exercise capacity, and actively cough and expectorate during the perioperative period, which can promote wound healing, reduce thrombotic disease, and reduce postoperative pneumonia and atelectasis. Several studies have reported the effectiveness of these measures during the perioperative period of lung cancer surgery (17-19). Leone et al. (17) found that tobacco abstinence contributed to improved patient-related outcomes at various phases of lung cancer management. The European Society for Clinical Nutrition and Metabolism (ESPEN) guidelines (18) highlight the nutritional aspects of the enhanced recovery after surgery (ERAS) concept and the special nutritional needs of patients undergoing major surgery. Another study (19) found that rehabilitation programs and exercise-based interventions can improve exercise capacity and lung cancer outcomes. Exercise capacity is prognostic and predictive of outcomes in lung cancer, and it is critical to risk-stratify patients undergoing evaluation for lung cancer resection. Thirdly, among these patients, 100 cases (78.74%) were IA stage NSCLC, while 107 cases (84.25%) underwent partial resection (including segmental and wedge resection), which requires less resection and involves less surgical trauma. A previous study (20) advocated sublobar resection for NSCLC patients with compromised cardiopulmonary reserve, as well as for selected patients with early-stage disease. Therefore, we also performed partial resection for early-stage NSCLC or patients with poor pulmonary function. Moreover, we routinely conduct intraoperative pathological examination of the 11th group of lymph nodes and margins, as well as its metastasis, to determine if we need to expand surgical resection or perform lobectomy.
In clinical practice, we found that when the tumor diameter was >5 mm, it could be easily detected by preoperative high-resolution CT. According to the 8th edition staging (13) of NSCLC, a tumor diameter of <10 mm is T1a, and most cases are adenocarcinoma in situ and minimally invasive adenocarcinoma or lepidic pattern-predominant adenocarcinoma; N2 lymph node metastasis rarely occurs in these types (21). When the lung tumor diameter is <5 mm, it is very difficult to locate, even under preoperative positioning in surgery, and so the surgical treatment of this kind of tumor is not recommended. However, when the tumor diameter is 5–10 mm, can accurately resect the tumors without changing the clinical stage of NSCLC. We found that the 100 patients (78.74%) with IA stage NSCLC had good prognosis during follow-up.
Doctor Qian has worked under Professor Li for 1 year at the Shanghai Chest Hospital, and has completed >100 lung tumor surgeries in his unit using CDIP. Compared to the preoperative invasive lung tumor positioning method, we believe that this method offers the following advantages. Firstly, it does not require expensive medical equipment and complex preoperative invasive localization, which many grassroots hospitals in China cannot implement due to economic and technical factors limitations and so on. Secondly, during the perioperative period, invasive methods can lead to some severe complications (5-7,22-24); most commonly, intrapulmonary focal hemorrhage (54.2%) (23), pneumothorax (7.5–49%) (5,6,22,23), chest pain requiring analgesia (9%) (5), hemosputum (2.6–6%) (5,22), dislocation of the marker (3.1–7.5%) (6,24), hemopneumothorax (0.6%) (5), and systemic air embolism (0.24%) (22). However, CDIP is used to locate the tumor during surgery, which can avoid the complications associated with invasive preoperative positioning. Furthermore, some patients have co-morbidities, including hypertension, diabetes, coronary heart disease, arrhythmia, cerebral infarction, etc., and preoperative invasive localization is also considered to be a high-risk operation in elderly patients. Moreover, preoperative invasive localization cannot be performed in some patients because of fear or physical condition, and thus, CDIP may benefit such patients. Thirdly, this approach can provide a reliable alternative when the marker is dislocated.
However, there are some disadvantages to CDIP. Firstly, the vertical distance and the clock dial position of tumors needs to be calculated according to the thoracic anatomy, which requires extensive clinical experience and good stereoscopic geometric imaging. Moreover, superficial lung tumors are relatively easy to locate, while deep lung tumor localization is extremely risk using this approach.
Conclusions
CDIP combined with SUPVATS is a safe, feasible, and effective method for the localization of lung tumors. This novel method can provide a reliable alternative technique for cases in which the marker is dislocated.
Acknowledgments
This project was supported by Yunnan Provincial Department of Science and Technology - Kunming Medical University Joint Special Project in 2019 under Grant (Detection of EGFR gene mutation and its clinical significance in non-small cell lung cancer in Dehong minority areas).
Funding: The research was supported by the joint project of Yunnan Provincial Department of Science and Technology and Kunming Medical University, China [2019FE001(-281)], and the scientific research project of Shanghai Municipal Commission of Health and Family Planning (20164Y0211).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/jtd-20-3312
Data Sharing Statement: Available at http://dx.doi.org/10.21037/jtd-20-3312
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd-20-3312). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Institutional Review Board and Ethics Committee of the Shanghai Chest Hospital of Shanghai Jiao Tong University. All patients granted their informed consent at least one day before surgery, following conscientious explanation of the procedure and the goals of the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018;68:7-30. [Crossref] [PubMed]
- Koike T, Togashi K, Shirato T, et al. Limited resection for noninvasive bronchioloalveolar carcinoma diagnosed by intraoperative pathologic examination. Ann Thorac Surg 2009;88:1106-11. [Crossref] [PubMed]
- Cox ML, Yang CJ, Speicher PJ, et al. The Role of Extent of Surgical Resection and Lymph Node Assessment for Clinical Stage I Pulmonary Lepidic Adenocarcinoma: An Analysis of 1991 Patients. J Thorac Oncol 2017;12:689-96. [Crossref] [PubMed]
- Wang GS, Wang Z, Wang J, et al. Uniportal complete video-assisted thoracoscopic lobectomy with systematic lymphadenectomy. J Thorac Dis 2014;6:1011-6. [PubMed]
- Watanabe K, Nomori H, Ohtsuka T, et al. Usefulness and complications of computed tomography-guided lipiodol marking for fluoroscopy-assisted thoracoscopic resection of small pulmonary nodules: experience with 174 nodules. J Thorac Cardiovasc Surg 2006;132:320-4. [Crossref] [PubMed]
- Ciriaco P, Negri G, Puglisi A, et al. Video-assisted thoracoscopic surgery for pulmonary nodules: rationale for preoperative computed tomography-guided hookwire localization. Eur J Cardiothorac Surg 2004;25:429-33. [Crossref] [PubMed]
- Sugi K, Kaneda Y, Hirasawa K, et al. Radioisotope marking under CT guidance and localization using a handheld gamma probe for small or indistinct pulmonary lesions. Chest 2003;124:155-8. [Crossref] [PubMed]
- Powell TI, Jangra D, Clifton JC, et al. Peripheral lung nodules: fluoroscopically guided video-assisted thoracoscopic resection after computed tomography-guided localization using platinum microcoils. Ann Surg 2004;240:481-8; discussion 8-9. [Crossref] [PubMed]
- Rho J, Lee JW, Quan YH, et al. Fluorescent and Iodized Emulsion for Preoperative Localization of Pulmonary Nodules. Ann Surg 2019. Epub ahead of print. [Crossref] [PubMed]
- Kuo SW, Tseng YF, Dai KY, et al. Electromagnetic Navigation Bronchoscopy Localization Versus Percutaneous CT-Guided Localization for Lung Resection via Video-Assisted Thoracoscopic Surgery: A Propensity-Matched Study. J Clin Med 2019;8:379. [Crossref] [PubMed]
- Hyun K, Park IK, Song JW, et al. Electromagnetic navigation bronchoscopic dye marking for localization of small subsolid nodules: Retrospective observational study. Medicine (Baltimore) 2019;98:e14831. [Crossref] [PubMed]
- Hsu PK, Wu YC. The feasibility of electromagnetic navigation-guided percutaneous microcoil localization for thoracoscopic resection of small pulmonary nodules. J Thorac Cardiovasc Surg 2019;157:e211-e214. [Crossref] [PubMed]
- Goldstraw P, Chansky K, Crowley J, et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2016;11:39-51. [Crossref] [PubMed]
- Lin JB, Qiu ML, Lin CJ, et al. Simplified synchronous disconnection of pulmonary arteries and veins for right upper lobectomy. Surg Endosc 2019;33:2015-23. [Crossref] [PubMed]
- Lin JB, Chen JF, Lai FC, et al. Transareolar pulmonary bullectomy for primary spontaneous pneumothorax. J Thorac Cardiovasc Surg 2016;152:999-1005. [Crossref] [PubMed]
- Nachira D, Meacci E, Porziella V, et al. Learning curve of uniportal video-assisted lobectomy: analysis of 15-month experience in a single center. J Thorac Dis 2018;10:S3662-S3669. [Crossref] [PubMed]
- Leone FT, Evers-Casey S, Toll BA, et al. Treatment of tobacco use in lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e61S-e77S.
- Weimann A, Braga M, Carli F, et al. ESPEN guideline: Clinical nutrition in surgery. Clin Nutr 2017;36:623-50. [Crossref] [PubMed]
- Ha D, Mazzone PJ, Ries AL, et al. The Utility of Exercise Testing in Patients with Lung Cancer. J Thorac Oncol 2016;11:1397-410. [Crossref] [PubMed]
- Altorki NK, Kamel MK, Narula N, et al. Anatomical Segmentectomy and Wedge Resections Are Associated with Comparable Outcomes for Patients with Small cT1N0 Non-Small Cell Lung Cancer. J Thorac Oncol 2016;11:1984-92. [Crossref] [PubMed]
- Cheng X, Zheng D, Li Y, et al. Tumor histology predicts mediastinal nodal status and may be used to guide limited lymphadenectomy in patients with clinical stage I non-small cell lung cancer. J Thorac Cardiovasc Surg 2018;155:2648-2656.e2. [Crossref] [PubMed]
- Ichinose J, Kohno T, Fujimori S, et al. Efficacy and complications of computed tomography-guided hook wire localization. Ann Thorac Surg 2013;96:1203-8. [Crossref] [PubMed]
- Lin MW, Tseng YH, Lee YF, et al. Computed tomography-guided patent blue vital dye localization of pulmonary nodules in uniportal thoracoscopy. J Thorac Cardiovasc Surg 2016;152:535-544.e2. [Crossref] [PubMed]
- Sui X, Zhao H, Yang F, et al. Computed tomography guided microcoil localization for pulmonary small nodules and ground-glass opacity prior to thoracoscopic resection. J Thorac Dis 2015;7:1580-7. [PubMed]
(English Language Editor: A. Kassem)


