
Introduction of robotic surgery leads to increased rate of segmentectomy in patients with lung cancer
Introduction
With more than 228,000 new cases annually, lung cancer is the leading cause of cancer-related death worldwide. Anatomic lung resection, including lobectomy and segmentectomy, is the mainstay of therapy for patients with early-stage non-small cell lung cancer (NSCLC). In appropriately selected patients, pulmonary segmentectomy leads to similar survival outcomes compared to lobectomy while avoiding removal of excess normal lung tissue (1,2). Therefore, segmentectomy may be advantageous in patients with minimal pulmonary reserve or compromised cardiac function.
Video-assisted thoracoscopic surgery (VATS) is utilized for a variety of thoracic procedures, however, this technique may not provide the optimal approach to segmentectomy. The limited, two-dimensional field of view as well as restricted instrument movements may prove challenging during distal isolation of segmental vessels and bronchi. The robotic surgical system provides theoretical advantages over VATS, including a three-dimensional field of view and improved dexterity. These benefits may allow for a more manageable learning curve compared to VATS and easier adoption of a minimally invasive technique (3-6). However, it remains unknown whether the advantages offered by robotic technology have enabled the pursuit of more technically challenging and complex thoracic procedures.
The purpose of this study was to evaluate whether the introduction of robotic thoracic surgery impacts the frequency of segmentectomies performed in patients undergoing minimally invasive anatomic lung resection for primary lung cancer. We hypothesized that the use of the robot would facilitate the performance of segmentectomy and promote the pursuit of parenchymal-sparing surgery in appropriately selected patients with small, early-stage lung tumors. We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/jtd-20-2249).
Methods
We conducted a single-institution retrospective review of adult patients undergoing a minimally invasive anatomic lung resection either by VATS or robotic-assisted approach between November 2017 and November 2019. We reviewed the electronic operating room schedule to identify patients scheduled for minimally invasive thoracic surgery during this time period and reviewed all medical records of eligible patients. Patient data including demographics, diagnosis, oncologic information, and operative details were collected from the patient’s medical record and stored in a REDCap database. We compared the proportion of VATS and robotic segmentectomies performed during the first year of the data collection period (November 1, 2017 to October 31, 2018) to the second year of the data collection period (November 1, 2018 to October 31, 2019). To mitigate selection bias, we compared histologic tumor type, segmental location of the tumor, and size of the tumor based on clinical T stage.
Operations were performed by the same four thoracic surgeons throughout the study period, with all surgeons participating equally in both VATS and robotic cases. The decision regarding operative approach was decided upon by the operating surgeon and patient following a collaborative discussion during the preoperative clinic visit. Options for operative technique and corresponding advantages and disadvantages of each approach were explained to the patient, however the final decision regarding VATS versus robotic approach was ultimately guided by the surgeon’s preference and expert recommendation.
A standard VATS technique was utilized for anatomic lung resection. Briefly, three trocars were placed in the hemithorax, including one for the video thoracoscope and two working ports. Single lung ventilation using a double-lumen endotracheal tube was employed. Using blunt dissection and electrocautery, the pulmonary vein was dissected free from its surrounding structures, encircled, and ligated with a stapler. The pulmonary artery was dissected in similar fashion and ligated with a stapler. The bronchus was similarly dissected using blunt dissection and electrocautery, followed by division with a stapler. The fissure between the lobes or lung parenchyma was then separated using multiple fires of the stapler to complete the lobectomy or segmentectomy, respectively. The robotic technique was performed in a similar fashion to the VATS approach with the exception of using five working ports instead of three.
Statistical analysis
Statistical analysis was performed using Prism 8 software (GraphPad Software Inc.). Data were compared using Fisher’s exact test or Chi-square test, where appropriate. A P value of <0.05 was considered statistically significant.
Ethical statement
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Approval for the study was obtained from the Colorado Multiple Institutional Review Board at the University of Colorado (protocol #19-2076). Individual consent for this retrospective analysis was waived.
Results
All cases
A total of 231 VATS and robotic anatomic lung resections were performed between November 2017 and November 2019. One hundred eighteen and 113 cases were performed during the first year and second year of the data collection period, respectively. Lung resections using a robotic-assisted approach comprised 15.3% of total cases the first year versus 78.8% of cases the second year (P<0.0001) (Figure 1A). Indications for surgical resection, including primary lung cancer, benign lung disease, and metastatic pulmonary nodule, accounted for 59.3%, 34.8%, and 5.9% of cases during the first year, respectively, versus 60.2%, 25.7%, and 14.2% of cases during the second year, respectively (P=0.06) (Figure 1B).
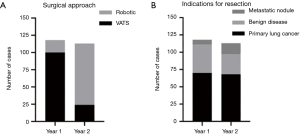
Primary lung cancer
A total of 138 VATS and robotic anatomic lung resections were performed for primary lung cancer. Seventy anatomic lung resections were performed during the first year, including 55 (78.6%) patients undergoing VATS resection and 15 (21.4%) undergoing robotic resection. During the second year, 68 cases were performed, including 14 (20.6%) using VATS and 54 (79.4%) using the robot. Types of lung cancer resected (adenocarcinoma, squamous cell carcinoma, or other), tumor size based on clinical T staging (T1–T4), and tumor location were not significantly different between years (P=0.44, P=0.98, and P=0.26, respectively). Segmentectomies performed for resection of a primary lung cancer included left upper lobe trisegmentectomy, lingulectomy, left lower lobe superior segmentectomy, left lower lobe basilar segmentectomy, right upper lobe apical segmentectomy, right upper lobe posterior segmentectomy, right lower lobe superior segmentectomy, and right lower lobe basilar segmentectomy. The proportion of segmentectomies increased from 8.6% during the first year to 25.0% during the second year (P=0.01) (Figure 2). Only patients with T1 tumors underwent segmentectomy. All segmentectomy specimens had negative margins on pathologic evaluation. One out of 6 (16.7%) segmentectomies were performed using the robot during the first year versus 15 out of 17 (88.2%) during the second year (P=0.003).
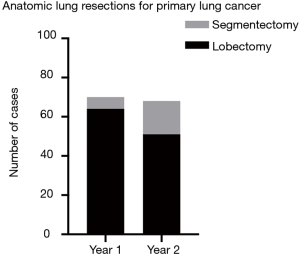
Discussion
For patients undergoing minimally invasive anatomic lung resection, the introduction of robotic thoracic surgery led to an overall increase in segmentectomy rate with an associated decrease in lobectomy rate. There was a statistically significant increase in the number of segmentectomies performed for patients with similarly staged NSCLC, the majority of which were performed robotically. In addition, an R0 resection was achieved in all segmentectomy cases. These results suggest that the introduction of robotic technology may curtail the limitations of traditional thoracoscopic techniques and help facilitate the performance of technically challenging procedures such as segmentectomy.
Historically, lobectomy was the treatment of choice for patients with early-stage NSCLC. This treatment recommendation is largely based on findings from a randomized trial performed by the Lung Cancer Study Group in 1995, which found improved survival and lower recurrence rate among patients undergoing lobectomy versus sublobar resection (wedge resection or segmentectomy) (7). However, the high number of wedge resections (32.8%) included in the sublobar resection group, lack of advanced imaging modalities, and questions surrounding proper preoperative staging limited the accurate comparison of outcomes among treatment groups.
Multiple studies have since challenged this treatment paradigm, reporting comparable outcomes following segmentectomy versus lobectomy for patients with small tumors without lymph node involvement (1,2,8-13). As a result, segmentectomy has become an accepted and attractive surgical option as it provides an adequate oncologic resection while preserving normal lung parenchyma. Preservation of lung is critical in elderly patients or those with low cardiopulmonary reserve in which unnecessary resection of normal lung tissue may decrease operative tolerability and negatively impact postoperative function. In addition, with increasing lung cancer awareness, improvements in imaging techniques, and widely available screening, a greater number of small, early-stage tumors are likely to be detected that are suitable for segmental resection. Yet, even for experienced thoracic surgeons, VATS segmentectomy can be a technically difficult procedure to perform.
There is a paucity of data regarding the impact of robotic surgery on the execution of challenging thoracic operations. A study by Kuo et al. prospectively evaluated the use of robotic-assisted surgery for 30 patients requiring complex thoracic operations that were formerly performed using an open approach (14). Included operations were those requiring difficult surgical dissections, complex sutures, or excision of tumors >8 centimeters. The authors concluded that robotic surgery provided a feasible and safe alternative to open surgery for these difficult procedures, particularly when performing complex sutures or removing large mediastinal tumors. A robotic approach has also been proven helpful for sleeve resections and thymic surgery, both of which are considered challenging procedures to perform using traditional VATS techniques (15,16).
There are several limitations of this study that warrant mention. The small case number and single-institution design limit the generalizability of the results. Additionally, the data must be interpreted with caution due to the methodological limitations associated with retrospective, observational studies. Additionally, once available, long-term oncologic follow-up data will provide valuable information to more adequately compare the efficacy of resection types. We also recognize the potential for selection bias given that surgeon experience and familiarity with particular operative techniques may influence frequency of application. However, in this study, all four surgeons had significant experience with VATS technique, whereas only one out of the four received formal fellowship training in robotic thoracic surgery. Therefore, upon establishment of our thoracic robotic program, three of the four surgeons had minimal to no experience using the robot. We would therefore expect these surgeons to preferentially choose VATS over a robotic approach, which we did not observe. Despite having significantly less experience with the robotic technique, surgeons opted to utilize the robot more frequently than VATS, even for seemingly more complex cases such as segmentectomies. This implies that familiarity with technique was less of an influence on frequency of application.
Conclusions
Utilization of the robotic platform has become increasingly widespread in thoracic surgery. However, robotic-assisted lung resection has failed to demonstrate a clear advantage over traditional VATS technique, with some studies reporting longer operative times and higher costs associated with the robot (17-20). The results of this study suggest that a robotic approach may help facilitate the performance of more complex minimally invasive procedures. By promoting easier execution of segmentectomies, robotic-assisted surgery may enable previously borderline operative candidates to undergo therapeutic pulmonary resection.
Acknowledgments
We thank Bryn Launer, John Olivas, and Michael Vrolijk for their help with data collection.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/jtd-20-2249
Data Sharing Statement: Available at http://dx.doi.org/10.21037/jtd-20-2249
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd-20-2249). CDS reports personal fees from Intuitive Surgical, Inc., outside the submitted work. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Approval for the study was obtained from the Colorado Multiple Institutional Review Board at the University of Colorado (protocol #19-2076). Individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bedetti B, Bertolaccini L, Rocco R, et al. Segmentectomy versus lobectomy for stage I non-small cell lung cancer: a systematic review and meta-analysis. J Thorac Dis 2017;9:1615-23. [Crossref] [PubMed]
- Bao F, Ye P, Yang Y, et al. Segmentectomy or lobectomy for early stage lung cancer: a meta-analysis. Eur J Cardiothorac Surg 2014;46:1-7. [Crossref] [PubMed]
- Marshall MB, Wee JO. Robotic Platform Use in General Thoracic Surgery. JAMA Surg 2019;154:1066-7. [Crossref] [PubMed]
- Arnold BN, Thomas DC, Bhatnagar V, et al. Defining the learning curve in robot-assisted thoracoscopic lobectomy. Surgery 2019;165:450-4. [Crossref] [PubMed]
- Petersen RH, Hansen HJ. Learning curve associated with VATS lobectomy. Ann Cardiothorac Surg 2012;1:47-50. [PubMed]
- Heemskerk J, van Gemert WG, de Vries J, et al. Learning curves of robot-assisted laparoscopic surgery compared with conventional laparoscopic surgery: an experimental study evaluating skill acquisition of robot-assisted laparoscopic tasks compared with conventional laparoscopic tasks in inexperienced users. Surg Laparosc Endosc Percutan Tech 2007;17:171-4. [Crossref] [PubMed]
- Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Lung Cancer Study Group. Ann Thorac Surg 1995;60:615-22; discussion 622-3. [Crossref] [PubMed]
- Ha KJ, Yun JK, Lee GD, et al. Surgical Outcomes of Radiographically Noninvasive Lung Adenocarcinoma according to Surgical Strategy: Wedge Resection, Segmentectomy, and Lobectomy. Korean J Thorac Cardiovasc Surg 2018;51:376-83. [Crossref] [PubMed]
- Nomori H, Mori T, Ikeda K, et al. Segmentectomy for selected cT1N0M0 non-small cell lung cancer: a prospective study at a single institute. J Thorac Cardiovasc Surg 2012;144:87-93. [Crossref] [PubMed]
- Tsutani Y, Miyata Y, Nakayama H, et al. Oncologic outcomes of segmentectomy compared with lobectomy for clinical stage IA lung adenocarcinoma: propensity score-matched analysis in a multicenter study. J Thorac Cardiovasc Surg 2013;146:358-64. [Crossref] [PubMed]
- Altorki NK, Yip R, Hanaoka T, et al. Sublobar resection is equivalent to lobectomy for clinical stage 1A lung cancer in solid nodules. J Thorac Cardiovasc Surg 2014;147:754-62; discussion 762-4. [Crossref] [PubMed]
- Landreneau RJ, Normolle DP, Christie NA, et al. Recurrence and survival outcomes after anatomic segmentectomy versus lobectomy for clinical stage I on-small-cell lung cancer: a propensity-matched analysis. J Clin Oncol 2014;32:2449-55. [Crossref] [PubMed]
- Koike T, Koike T, Sato S, et al. Lobectomy and limited resection in small-sized peripheral non-small cell lung cancer. J Thorac Dis 2016;8:3265-74. [Crossref] [PubMed]
- Kuo SW, Huang PM, Lin MW, et al. Robot-assisted thoracic surgery for complex procedures. J Thorac Dis 2017;9:3105-13. [Crossref] [PubMed]
- Cerfolio RJ. Robotic sleeve lobectomy: technical details and early results. J Thorac Dis 2016;8:S223-6. [PubMed]
- Bodner J, Wykypiel H, Wetscher G, et al. First experiences with the da Vinci™ operating robot in thoracic surgery. European Journal of Cardio-Thoracic Surgery 2004;25:844-51. [Crossref] [PubMed]
- Augustin F, Bodner J, Maier H, et al. Robotic-assisted minimally invasive vs. thoracoscopic lung lobectomy: comparison of perioperative results in a learning curve setting. Langenbecks Arch Surg 2013;398:895-901. [Crossref] [PubMed]
- Park BJ, Flores RM. Cost comparison of robotic, ideo-assisted thoracic surgery and thoracotomy approaches to pulmonary lobectomy. Thorac Surg Clin 2008;18:297-300. vii. [Crossref] [PubMed]
- Lee BE, Korst RJ, Kletsman E, et al. Transitioning from video-assisted thoracic surgical lobectomy to robotics for lung cancer: are there outcomes advantages? J Thorac Cardiovasc Surg 2014;147:724-9. [Crossref] [PubMed]
- Swanson SJ, Miller DL, McKenna RJ Jr, et al. Comparing robot-assisted thoracic surgical lobectomy with conventional video-assisted thoracic surgical lobectomy and wedge resection: results from a multihospital database (Premier). J Thorac Cardiovasc Surg 2014;147:929-37. [Crossref] [PubMed]


