Big-data Clinical Trial (BCT): the third talk
In the previous talk, the randomized control trial (RCT) showed less possibility to keep its major role in the future clinical studies (1). Meanwhile, the concept of “Big-data Clinical Trial (BCT)” was discussed and redefined in the following articles (2,3). In this article, we will continue the topic on BCT from the perspective of “data structure”. We hope BCT will have an adequate arena to take over RCT and win the “relay race” in the upcoming new era.
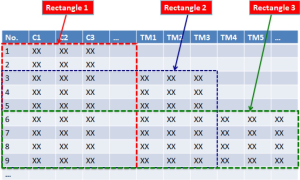
In “Big Data”, we have 4Vs: Volume, Velocity, Variety, Veracity, and there could be three requirements for the clinical data in BCT. Firstly, the Volume of data should be “big” enough, even it is mined from a single case: the data would cover the whole process of health care including the diagnosis (e.g., genome-wide findings instead of selected markers), treatment (e.g., real-time surgical videos instead of intra-operative still pictures), and results (e.g., life-long instead of 3 or 5 year follow-ups); secondly, the Velocity of the data refreshment would be “high” enough to realize the real-world evidence studies; thirdly, the data would have Variety in structure, and new parameters would be continuously introduced to the study, resulting in a “ladder-shaped” data structure (Figure 1). The expanding parameters of the study stand for the increasing human knowledge on diseases; even the information is temporally compromised in Veracity.
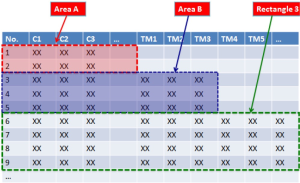
Currently, most clinical research articles are based on the statistical analyses on the “rectangle-shaped” data. In Figure 1, for instance, the first column represents the numbered individuals (1, 2, 3..), and the rows shows various parameters including sex and age (the same parameters apply each individual, as shown in rectangles 1, 2, and 3 in Figure 1). Suppose we carry out statistical analysis on data in rectangle 3 (Figure 2), data in areas A and B will not be included. The question is: Should data in area A and/or area B be included for analyses?
As was suggested in a recent TED talk by Kenneth Cukier: three additional parameters were discovered in determine the malignancy from breast specimens by data scientist, but not the pathologist! In BCT, the continuous expansion of parameters would probably summon the beast called “Machine Learning”, and the future of medicine would welcome the collaboration between physician and data scientist.
All in all, there will be long journey before the human beings realized the “Precision Medicine” from BCT. Long as it is, soon it will be.
Acknowledgements
Supported by the American Association for Thoracic Surgery’s Evarts A. Graham Memorial Traveling Fellowship; and the National Natural Science Foundation of China (Grant No. 81400681).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Wang DY. RCT and “relay race”. Available online: http://kysj.amegroups.com/articles/1144
- Wang SD. Opportunities and challenges of clinical research in the big-data era: from RCT to BCT. J Thorac Dis 2013;5:721-3. [PubMed]
- Wang SD, Shen Y. Redefining big-data clinical trial (BCT). Ann Transl Med 2014;2:96. [PubMed]