AME College: a Clinical Research Training Program
Scientific research brings the perspective of a third eye to clinicians. For each young doctor who is aspiring to become an excellent clinician, it is a must. However, the promotion and conduct of clinical research in China is far from satisfactory due to various reasons. While many Chinese clinicians have accumulated rich clinical data, they are technically not able to design good clinical studies; meanwhile, they face many challenges to publish their findings in high-profile SCI journals. The vast majority of clinical evidences currently are based on foreign clinical trials. Thus, we initiated this “AME College: Clinical Research Training Program” with a focus to promote the clinical research-related knowledge. Our training Course s will include, but not limited to, the following topics:
- Course 1: clinical research design and registration of research protocols (including writing of trial protocols, collection of trial data, and management and statistical analysis of research data);
- Course 2: applied statistics in medical research (including the selection of feasible statistical methods and its realization in statistical software);
- Course 3: skills for writing and publishing an article in a SCI-indexed journal (including manuscript writing, table making, image processing, manuscript submission, and communication with editors/peer-reviewers);
- Course 4: access to evidence-based medicine (EBM) resources and critical reading of evidences (including clinical evidence retrieval and a thorough understanding of the results of various clinical studies);
- Course 5: methodologies of systematic review/meta-analysis (methodological training sessions for beginners and intermediates);
- Course 6: advanced methodologies of systematic review/meta-analysis (methodological training sessions for advanced trainees).
The delicately designed “AME College: Clinical Research Training Program” will be officially launched in August 2015. With strong supports from the AME lecturer panel both at home and abroad, our training program is dedicated to bringing the most practical clinical research skills and know-hows to our trainees and helping them to avoid any unnecessary mistake or error. We hope our efforts will help most Chinese clinicians to master the methodologies in clinical research, design more high-quality clinical trials, and make more voices from China be heard in top medical journals. Fighting!
Course planning and description
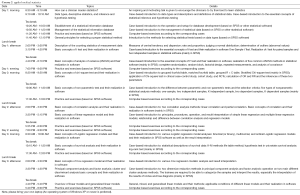
Clinical study design/applied medical statistics/tips for writing a scientific article
Scientific research is just like another eye, leg, or arm for a clinician. For each young doctor who is aspiring to become an excellent clinician, it is a must. However, in the real world, a large proportion of Chinese clinicians still conduct clinical research in an inappropriate or even wrong way of thinking. For example, the common mistakes or errors during clinical trial design include ambiguous inclusion criteria, inappropriate case selection, confusion between univariate and multivariate analysis, and inaccurate estimation of sample size. Also, in some studies, the recruited groups are imbalanced at baseline; the authors may arbitrarily set control group, regarding “arbitrariness” as “randomization”. Furthermore, the blinding is lack or inappropriate, the description of data collection is not appropriate, and, the statistical analytic method is misused.. All these problems not only hinder a clinician’s career development but also do harm to the development of medical sciences in China. In addition, some studies also have found that up to one third of research findings published in top medical journals including N Engl J Med, JAMA, and Lancet failed to stand the test of time. Due to the lack of knowledge and skills in writing scientific articles, many clinicians failed to publish their important clinical findings; as a result, their academic achievements cannot be recognized by international colleagues and many valuable clinical data become useless. It’s like we caught good cards, but lost the game for not playing well. Therefore, it is particularly important to constantly carry out training on scientific research for clinicians.
Clinicians are always busy. How to help them to quickly identify a research topic, reasonably carry out research design, and properly select statistical analysis methods, and finally successfully publish their findings in a high-profile SCI journal has become an urgent and realistic task. Thus, AME launched this AME College with a target to carrying out training Course s on clinical research design/applied medical statistics/scientific writing skills in major cities and collaborating hospitals across China. Compared with other training Course s, the AME College has many features: all the Course s are specifically designed for clinicians; statistics will be a training priority since it remains a challenge for most clinicians; and tailored training will be performed on the common mistakes/errors found in scientific articles written by Chinese doctors. The college will be launched by AME Publishing Company. Experts with rich “real world” experiences in clinical research design/applied medical statistics/scientific writing skills from China and abroad will be invited to teach in the training Course s. Based on detailed true cases, they will share their own experiences through interactive learning modes including lectures, discussions, and exercises. Teaching assistants (TAs) will be also available to help the trainees to complete their exercises. All the Course s are designed to be practical and simple. Furthermore, efforts have been made to ensure the curriculum design and teaching methods meet the thinking patterns of Chinese clinicians; in other words, the training content must be understandable, acceptable, and repeatable. The ultimate mission of AME College is to serve Chinese clinicians and promote the development of medical sciences in China.
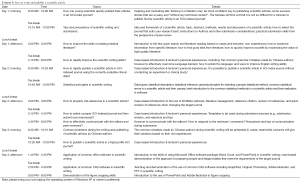
Evidence-based medicine (EBM) and systematic review/meta-analysis training Course s
EBM is an interdisciplinary approach emerging from 1980s that integrates clinical medicine, epidemiology, and statistics. As an applied discipline with a mission to address the challenges in health care, it remains one of the most important and active disciplines in medical sciences. Systematic review/meta-analysis is one of the key EBM methodologies that produce high-quality evidences. A solid understanding and proper application of systematic review/meta-analysis will help clinicians to deeply understand clinical epidemiology and statistics, enable them to correctly interpret the results of clinical studies published in medical journals, and facilitate the clinicians to improve their capabilities in clinical research design and in writing and publishing high-profile scientific articles. In return, it is also an integral part of clinical competence for clinicians.
The value of systematic review/meta-analysis has increasingly been recognized by Chinese clinicians in the past decade. The number of systematic review/meta-analysis written by Chinese authors has increased annually in the SCI database. Today, the number of systematic review/meta-analysis authored by Chinese scholars ranked fourth worldwide, surpassed only by the United States, the United Kingdom, and Canada. Although the number of Meta analyses has been large, their quality remains suboptimal. Few meta-analysis authored by Chinese scholars have high impacts. In addition, these articles often have much room to improve in terms of methodologies, reporting standards, and writing skills. Therefore, we invited top Chinese scholars with rich experiences in systematic review/meta-analysis to thoroughly explain the methodologies of systematic review/meta-analysis and share their skills and experiences in writing and publishing these articles. It is expected that this Course will help to improve Chinese clinicians’ capabilities in systematic review/meta-analysis and help Chinese authors to publish scientific articles with higher impacts, so to as to promote the development and application of EBM in China.
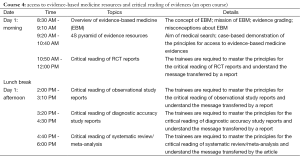
Focusing on the preparation of systematic review, this Course will include: an overview of EMB and systematic review; proposing a clinical research topic; statistical issues in meta-analysis; strategies and methods for searching EBM literature; quality evaluation for common clinical reports; systematic review/meta-analysis on interventions, diagnosis, etiologies, and prognostic factors; application of software such as Revman (version 5.3) and Stata (version 13.0) in meta-analysis.
Features: this Course is designed to improve the trainees’ actual capabilities in systematic review/ meta-analysis by focusing on the following issues: what is systematic review/meta-analysis? How to choose a topic? How to search related literature? How to conduct literature quality evaluation? How to retrieve data from the yielded literature? How to analyze and interpret data using software? What is the core process for writing a systematic review? Without any didactic and pedantic talks, the Course is highly practical and will be given in case-based teaching workshop, whenever possible.
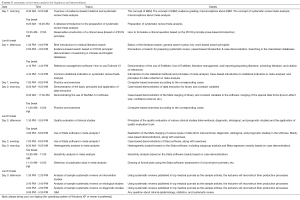
Advanced methodologies of systematic review/meta-analysis
Along with the wide spread of the knowledge of systematic review/meta-analysis, a growing number of clinicians have become interested in the advanced methodologies of systematic review/meta-analysis. In fact, the methodologies of meta-analysis have rapidly developed in recent years, as the traditional meta-analysis methods have shown many limitations when addressing some clinical issues. For instance, in the randomized controlled trials (RCTs), it is difficult to directly compare multiple different interventions targeting the same clinical condition. However, the users of the evidences are often eager to distinguish these interventions; then, how can such indirect comparisons be achieved by using a proper meta-analysis method? Also, for data describing dose–response relationships in etiological studies, can we merge them? These issues need to be addressed using non-classical meta-analysis methods. According to our tracing of systematic reviews/Meta analyses published in top medical journals, the acceptance of these articles is not only because the research topics are important but also because the authors had adopted more sophisticated meta-analysis methodologies such as meta-analysis based on individual patient data (IPD) or network meta-analysis. Therefore, in our training Course on the advanced methodologies of systematic review/meta-analysis, we will provide a good platform for trainees interested in systematic review/meta-analysis to share their knowledge and skills, so as to standardize and promote the application and development of advanced methodologies of systematic review/meta-analysis in China.
Again, this training Course is designed to meet the real needs of trainees. The 3-day training Course will cover topics including network meta-analysis methodologies, meta-analysis of rate, meta-analysis of count data, meta-analysis of dose-response relationships, meta-analysis of IPD, and meta-analysis of survival data. Again, with an attempt to increase the trainees’ capability in conducting systematic review/meta-analysis, a case-based teaching mode will be adopted, along with the personal experiences shared by the lecturers. An open teaching mode integrating lectures, demonstrations, exercises, and discussions will be applied. Also, the trainees will be arranged to receive computer-based training and user data communication sessions, focusing on the usage of Stata, WinBUGS, R language, and ADDIS software, which main involves data processing, interpretation of program codes, drawing of network maps, and presentation of analysis results. Again, efforts will be made to ensure the training content is understandable, acceptable, and repeatable.
Course schedule

Full table
Full table
Full table
Full table
Full table

Full table
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.


