Diagnosis of systemic amyloidosis and amyloidosis mediated cardiomyopathy by VATS pleural biopsy for chronic pleural effusion
Introduction
Amyloidosis is characterized by extracellular accumulation of amyloid protein into organs leading to such pathologies as renal failure and cardiomyopathy. Pleural effusions are a rare sequela of amyloidosis only occurring in 6-18% of cases (1). Amyloid mediated cardiomyopathy is a poor prognostic indicator of disease progression. Endomyocardial biopsy is the gold standard of diagnosis for patients with systemic amyloidosis (2). We present a novel case, where the diagnosis of systemic amyloidosis was made by video-assisted thoracic surgical (VATS) pleural biopsy after a false negative endomyocardial biopsy.
Case report
A 77-year-old male with a history of idiopathic congestive heart failure and chronic pleural effusions presented with shortness of breath and malaise. Despite repeated thoracentesis and diuretic treatment, he continued with symptomatic pleural effusions.Each of his thoracentesis procedures temporarily relieved his shortness of breath. Chemical and cytologic analysis of the fluid was repeatedly non-diagnostic. A chest radiograph on admission to our institution demonstrated a large left hydrothorax with only a small pleural effusion on the right.
A transthoracic echocardiogram demonstrated an ejection fraction of 35-45% and severe concentric left ventricular (LV) hypertrophy with an intraventricular septum of 2.0 cm and LV posterior wall of 1.9 cm. The patient was managed medically for optimization of his congestive heart failure. Twenty four hour urine collection demonstrated a high protein level of 2.3 mg/dL (reference range <150 mg/dL per twenty four hours) and electrophoresis showed a monoclonal protein present at 3.0 mg/dL.
An 8.5 French left pleural drain was placed for drainage of the large left pleural effusion. Pleural fluid was without malignant cells, did not grow organisms, and was found to be transudative in nature (pleural fluid protein 1.9 g/dL, serum protein 6.2 g/dL). Despite aggressive diuresis and fluid restriction, fluid drainage was as high as 750 cc in a twenty four hour period.
A cardiac catheterization was performed and endomyocardial biopsy collected. Results of the cardiac catheterization were suggestive of restrictive cardiomyopathy. Endomyocardial biopsy of the right ventricle initially demonstrated myocardial fiber loss with diffuse interstitial fibrosis. A Congo red stain was negative for amyloid. Thoracic surgery was consulted for pleural tissue acquisition and definitive therapy.
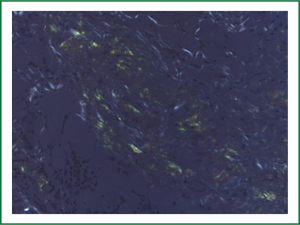
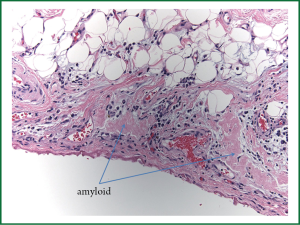
The patient underwent a fiberoptic bronchoscopy as well as a left VATS pleural biopsy and talc pleurodesis. 600 cc of straw colored pleural fluid was removed from the left pleural space. The lung parenchyma was noted to be hyperemic. No pleural nodules were visualized. Pathology reported that the Congo red stain was positive for amyloid deposition in the pleural tissue, as well as abundant amyloid deposits on light microscopy (Figures 1,2). After 72 hours, the chest tube was removed with minimal output and resolution of the pleural effusion. The patient underwent a bone marrow biopsy that showed an abnormal population of Serum amyloid A (AL) lambda monotypical plasma cells consistent with amyloidosis. The endocardial specimen was re-reviewed using liquid chromatography tandem mass spectrometry, and was found to have a peptide profile consistent with AL lambda-type amyloid deposition.
The patient was started on a regimen of bortezomib, a proteasome inhibitor. The patient expired two and a half months after his biopsy and pleurodesis. He did not require repeat drainage of the left pleural effusion before his death.
Discussion
Amyloidosis is a family of diseases that is characterized by extracellular accumulation of bent proteins that are shaped as fibrils. This misfolding into beta pleated sheets accounts for both its unique staining and microscopic visual appearance(3). Amyloid is histologically described as congophilic, meaning that under polarized electron microscopy it presents green birefringence when dyed with Congo red stain(4). Deposition of amyloid protein into extracellular sites is the etiology of its pathogenesis; leading to nephrotic syndrome, renal failure, peripheral neuropathies or congestive heart failure based on the organ of aggregation.
There are both systemic and local forms of amyloidosis. The most common form of amyloidosis, primary systemic amyloidosis, is known as serum amyloid A or AL. AL is a plasma cell dyscrasia in which the amyloid protein is composed of immunoglobulin light chains, specifically monoclonal κ or λ chains. Pleural effusions are a rare sequela of AL. About 6-18% of cases AL are complicated by pleural effusion though the mechanism for the pleural effusions in AL is unknown(1,5-7). Cardiomyopathy is a negative prognosticator of systemic amyloidosis. Endomyocardial biopsy is the gold standard test for diagnosis of cardiac amyloidosis, however it is not without complications and four biopsy specimen are required to increase the sensitivity (2). This case report illustrates the difficulty in diagnosing systemic amyloidosis, especially in the setting of an initial nondiagnostic endomyocardial biopsy. The uniqueness of this case highlights a diagnosis being made by VATS pleural biopsy after a false negative Congo red staining of an endomyocardial biopsy. The positive VATS pleural biopsy, led to reanalysis of the endomyocardial specimen by mass spectrometry confirming the pathologic diagnosis.
Studies show that neither the degree of heart failure nor nephrotic syndrome can account for the pleural effusions; but the actual infiltration of amyloid into the pleural tissue may be the etiology of the pleural effusions (8).
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Berk JL. Pleural effusions in systemic amyloidosis. Curr Opin Pulm Med 2005;11:324-8. [PubMed]
- Kapoor P, Thenappan T, Singh E, et al. Cardiac amyloidosis: apractical approach to diagnosis and management. Am J Med 2011;124:1006-15. [PubMed]
- Kyle RA. Amyloidosis. Circulation 1995;91:1269-71. [PubMed]
- Picken MM, Pelton K, Frangione B, et al. Primary amyloidosis A. Immunohistochemical and biochemical characterization. Am J Pathol 1987;129:536-42. [PubMed]
- Mansalis KA, Klein DA, Demartini SD, et al. Pleural findingsin a patient with persistent pulmonary effusions from systemic amyloidosis. Amyloid 2011;18:29-31. [PubMed]
- Bontemps F, Tillie-Leblond I, Coppin MC, et al. Pleural amyloidosis: thoracoscopic aspects. Eur Respir J 1995;8:1025-7. [PubMed]
- Briggs JH, Singleton WG, Burke MM, et al. Amyloidosis presenting as bilateral transudative pleural effusions with normal cardiac investigations: acase report. Cases J 2009;2:6963. [PubMed]
- Berk JL, Keane J, Seldin DC, et al. Persistent pleural effusions in primary systemic amyloidosis: etiology andprognosis. Chest 2003;124:969-77. [PubMed]