New developments in the use of positive airway pressure for obstructive sleep apnea
Introduction
Obstructive sleep apnea (OSA) is a disorder characterized by intermittent reduction of breathing due to complete or partial occlusion of the upper airway during sleep (1). The prevalence of OSA has been rising over the last several decades in part due to the obesity pandemic. It is estimated that 13% of men and 6% of women have moderate to severe OSA defined as an apnea-hypopnea index (AHI) ≥15/hour (2).
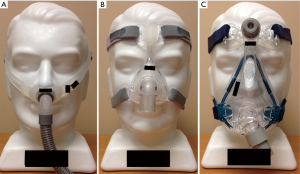
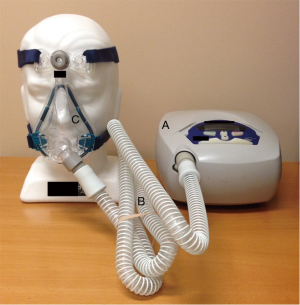
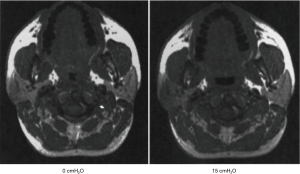
First developed in the early 1980’s (3), continuous positive airway pressure (CPAP) has become established as the treatment of choice for OSA (4). CPAP consists of a mask through which airflow is delivered by a blower (Figure 1). This servo-controlled fixed pressure is designed to overcome the tissue forces tending to collapse the upper airway thereby stenting the airway open. Because of this concept, CPAP is commonly referred to as a ‘pneumatic splint’ (Figure 2) (3).
CPAP as a treatment of OSA symptoms
A large literature exists demonstrating the efficacy of CPAP in treating OSA symptoms. Snoring, a very common presenting complaint, represents turbulent airflow caused by upper airway narrowing (6). Snoring can be bothersome to the bed partner adversely impacting their sleep quality and can lead to a loss of intimacy when it causes the patient and partner to sleep apart. In addition, vibration of the carotid arteries due to snoring may theoretically increase stroke risk independent of airway obstruction or hypoxemia (7). By preventing airway collapse/vibration, CPAP eliminates snoring (8). As a result, CPAP has been shown to improve sleep quality in the bed partner (9). Similarly, evidence suggests CPAP can reduce other nocturnal symptoms such as gasping or choking, nocturnal awakenings, and nocturia (10).
Another cardinal symptom of OSA is excessive daytime sleepiness (11,12). Randomized controlled trials have consistently demonstrated that CPAP improves sleepiness as assessed both objectively and subjectively (13). Compared to sham CPAP, one large meta-analysis found CPAP reduces sleepiness on the Epworth Sleepiness Scale (ESS) by an average of 4 points (13). Studies suggest that even in mild OSA associated with sleepiness, CPAP can improve symptoms (14). A major consequence of excessive daytime sleepiness is motor vehicle collisions. Observational studies suggest that CPAP therapy improves performance on driving simulator tasks (15), and CPAP therapy is associated with a decline in motor vehicle accident rate (16).
Another symptom commonly attributed to OSA is depressed mood. Results of clinical trials assessing the impact of CPAP on mood have been mixed with both positive and negative results. A limitation of the literature has been the lack of studies focusing on OSA patients with depressed mood at baseline (13). OSA may also affect cognitive function; however, the Apnea Positive Pressure Long-term Efficacy Study (APPLES), the largest randomized trial in OSA done to date, did not demonstrate any sustained improvements in cognitive function despite detailed testing in multiple domains. Criticisms of this trial were the large number of dropouts and that the enrolled population had normal cognitive function at baseline (17). Whether CPAP improves function or prevents decline in those with cognitive impairment at baseline is unclear, although a small randomized trial in patients with Alzheimer’s dementia suggested potential benefits (18,19).
Furthermore, CPAP has consistently been shown to produce improvements in OSA-specific quality of life measures (20,21). However, when assessed using global quality of life measures such as the 36-Item Short Form Health Survey (SF-36), a meta-analysis of eight studies suggests the impact of CPAP on global quality of life is relatively small, with the greatest benefit in the domains of physical function, general health, and vitality (13).
CPAP to improve long term outcomes
In addition to symptoms, OSA is associated with a host of cardiovascular risk factors and adverse outcomes including hypertension, stroke, and heart failure, leading many clinicians to recommend treatment of OSA even in asymptomatic patients. Among the OSA treatments available, CPAP has the strongest evidence for a beneficial cardiovascular effect (22-30).
OSA has been well established as an independent risk factor for hypertension (31,32). Randomized trials show a small but consistent effect of CPAP therapy in lowering blood pressure. Based on a large meta-analysis, CPAP lowers systolic blood pressure (SBP) by 2.5 mmHg and diastolic blood pressure (DBP) by 1.8 mmHg (33). Of note, increased CPAP adherence predicts greater blood pressure reductions. In one trial, SBP and DBP fell by <1.5 mmHg in those using CPAP ≤5.65 hours per night, but fell by 3.7 and 5.6 mmHg respectively in those with >5.65 hours of usage (22). Some studies have found minimal to no effectiveness of CPAP therapy on blood pressure and other vascular measures in non-sleepy adults (22,34-36). However, a meta-analysis using individual patient data from these four studies demonstrated improvement in DBP (−1.4 mmHg) in those using therapy for >4 hours/night. These data would suggest that the lack of effect may be secondary to non-adherence, but biological variations in susceptibility to OSA consequences may also be important (37). It has also been postulated that the blood pressure response to CPAP may be greatest in those with resistant hypertension as a small trial reported 6.5±3.3 and 4.5±1.9 mmHg drops in daytime SBP and DBP respectively (38). However, a larger trial found the 24-hour mean arterial pressure reduction was only 3.1 mmHg, similar to the magnitude in other hypertension studies (39).
In addition to hypertension, OSA is also independently associated with other components of the metabolic syndrome such as impaired glucose tolerance (40) and dyslipidemia (41,42). In clinical trials, CPAP has not been shown to improve fasting glucose in normoglycemic patients but has improved measures of insulin resistance on challenge testing (43-45). In a trial of patients with impaired glucose tolerance at baseline and moderate to severe OSA, CPAP did not improve glucose tolerance overall but there was evidence of improvements in post-hoc exploratory analyses among those with severe OSA at baseline (46). Finally, a small trial among patients with type 2 diabetes showed no improvement in glucose control with CPAP therapy (47). Regarding lipid levels, trials have been consistently negative in demonstrating an improvement in fasting lipid levels with CPAP (43,48,49). However, a small trial suggested 24-hour levels of triglycerides improve with CPAP therapy due to improvements in post-prandial levels (50). Several trials have assessed the impact of CPAP on visceral fat. Overall, these studies have not shown an impact of CPAP on visceral fat mass (45,51,52). However, there does appear to be an effect of CPAP on weight such that CPAP therapy is associated with a small but consistent increase in weight (53-55). Some have suggested this finding is due to reduced work of breathing at night though others have argued this observation represents an increase in muscle mass due to improvements in growth hormone function (56). Another possibility is that CPAP restores normal social activities which often involve caloric intake (e.g., dinner with spouse, beers with friends) leading to weight gain.
Data from the Sleep Heart Health Study have demonstrated between OSA and incident cardiovascular disease including stroke, coronary artery disease, and heart failure (25,29). The relationship of CPAP to stroke prevention remains unclear, but among stroke survivors with OSA, CPAP improves neurologic and motor recovery after stroke, delays appearance of cardiovascular events, and may prevent cardiovascular events (57,58). In observational studies, CPAP users experience a lower incidence of fatal and non-fatal coronary events compared to non-users (23,27). A large randomized trial conducted by the Spanish Sleep and Breathing Network did not demonstrate a reduction in the combined endpoint of incident hypertension and cardiovascular events in people with asymptomatic OSA, although this trial may have been underpowered (22). Several larger randomized trials are currently underway to address more definitively the impact of CPAP on cardiovascular event risk. These include the Sleep Apnea Cardiovascular Endpoints (SAVE) study, the Randomized Intervention with CPAP Treatment in Coronary Artery Disease and Sleep Apnea (RICCADSA) trial, and the Continuous Positive Airway Pressure in Patients with Acute Coronary Syndrome and Obstructive Sleep Apnea (ISAACC) trial (59,60).
In short term trials, CPAP has been shown to improve left ventricular ejection fraction in patients with OSA and systolic heart failure (61,62). In patients with OSA and heart failure with preserved ejection fraction, CPAP use improves diastolic function (63). In addition, observational studies suggest CPAP use in OSA and heart failure is associated with a lower risk of death and hospitalization (64,65). In terms of pulmonary hypertension, a small randomized crossover trial found CPAP led to a 4.9 mmHg reduction in pulmonary artery systolic pressure (66). A number of studies have also evaluated the role of CPAP in the prevention of atrial fibrillation. Observational studies have demonstrated that patients with treated OSA have approximately half the rate of recurrent atrial fibrillation following ablation compared to those with untreated OSA (67-69). However, because these results may be due to fundamental differences predicting adherence (“healthy user effect”), randomized studies in this population are needed.
Observational studies suggest that people with OSA also may have higher rates of incident cancer and cancer-related mortality (70,71). Clinical outcome data suggest individuals who use CPAP may have lower mortality compared to those who are untreated (72,73).
Interventions to improve adherence
Although CPAP is established as a highly efficacious treatment for OSA, its effectiveness has been limited by poor adherence. Users often experience nasal discomfort, congestion, mask leak and claustrophobia which lead to variable levels of long term compliance ranging from 46% to 85%, depending on how compliance is defined (74). Although adherence has been arbitrarily defined as usage for >4 hours/night more than 70% of nights for the purposes of insurance reimbursement in the United States, there does not appear to be a clear threshold above which adverse effects of OSA reverse (75). In contrast, there is a fairly linear dose response relationship such that the greater the CPAP usage, the greater the improvement in sleepiness, quality of life, or blood pressure outcomes (22,27). As a result, there has been much research on methods to optimize CPAP adherence.
Device manufacturers have developed advances in PAP technology as one means to improve acceptance and adherence. Advanced PAP features include auto-titrating PAP (APAP), bi-level PAP (BPAP), and expiratory pressure relief (EPR). A goal for all of these interventions has been to lower the pressure delivered to the airway due to the concern that higher pressures lead to reduced patient adherence (Figure 3). However, most observational data have not identified PAP level as a predictor of adherence (76,77).
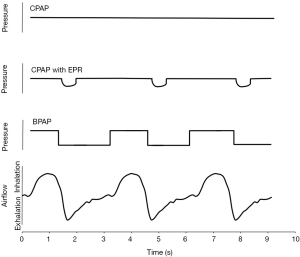
Bi-level positive airway pressure (BPAP)
BPAP was the first device developed to try to lower mean airway pressures.
Instead of applying a fixed pressure throughout the respiratory cycle, BPAP applies a lower expiratory positive airway pressure (EPAP) during exhalation and a higher inspiratory positive airway pressure (IPAP) during inhalation (78,79). By maintaining IPAP above Pcrit, the EPAP may be reduced without airway collapse. This approach can lower mean airway pressure particularly during exhalation when the patient has to breathe out against the delivered pressure. In the only large comparative trial evaluating BPAP with CPAP in PAP-naïve OSA (n=83), nightly usage between the two groups was similar as were the range and number of complaints. In fact, BPAP users with a large (greater than 6 cmH2O) IPAP-EPAP difference had significantly lower compliance than CPAP users (80). Nevertheless, BPAP may be helpful in the subset of patients who complain of pressure intolerance. In addition, because BPAP machines can generate pressures above the maximal CPAP level of 20 cmH2O, BPAP can be useful in the most severe OSA patients. Finally, use of a high IPAP to EPAP difference can be used to increase the tidal volume and so provide ventilatory support in people with hypoventilation syndromes (80). Retrospective data from a population of US Military Veterans indicate that BPAP is used more often in individuals with high BMI, CHF, COPD, hypercapnia, and severe hypoxia (81).
Auto-titrating devices
Instead of operating at a set pressure, APAP monitors a patient’s respiratory activity in order to provide the lowest level of PAP necessary to eliminate respiratory disturbances (82). Algorithms are designed to increase pressure when events are noted and to decrease pressure slowly if events have not occurred for a period of time. Because the minimum PAP level necessary to eliminate airway obstruction varies over the night by position, sleep stage, and other factors, APAP has the capacity to provide the lowest pressure necessary at each time and therefore lower the overall mean pressure across the night (83). Compared to CPAP, APAP demonstrates a small but statistically significant superiority in adherence (+11 minutes) as well as in reduction of sleepiness (−0.5 points in ESS) (84,85). In contrast, evidence suggests that fixed CPAP level may be superior to APAP in blood pressure reduction and other cardiometabolic outcomes (85). For now, we regard fixed CPAP as the treatment of choice based on available data.
Another use of APAP has been to determine CPAP level requirements in the home rather than a sleep laboratory. Typically, the patient is provided an APAP device for 5-7 nights and the device is then interrogated to identify the 90th or 95th percentile pressure required which is inferred to be their fixed CPAP requirement. Such a protocol has been demonstrated to identify CPAP requirements effectively. A diagnostic protocol combining home sleep testing followed by APAP titration has been shown to reduce costs of OSA diagnosis and treatment substantially, lower time to treatment, and improve CPAP adherence as compared to the traditional strategy of in-laboratory polysomnography and CPAP titration (86).
It is important to note that each APAP manufacturer has their own proprietary algorithm for determining pressure changes and the optimal algorithm is unclear. Auto-titrating algorithms have also been developed for BPAP and a small study suggests auto-titrating BPAP may be helpful as a rescue therapy for occasional patients failing CPAP, although in aggregate the trial was negative (87).
Expiratory pressure relief (EPR)
Another strategy to improve compliance has been the use of EPR. Similar to BPAP, machines programmed to perform pressure relief provide less airway pressure during early exhalation compared to standard PAP at the same set pressure. Unlike BPAP, EPR varies with each breath according to the expiratory flow rate—a higher expiratory flow rate from the patient yields a greater EPR resulting in a greater pressure drop in early exhalation as opposed to late exhalation. An initial non-randomized evaluation found an impressive improvement in CPAP adherence (~1.5 hours/night) with EPR (88). However, five randomized trials have been subsequently performed and a meta-analysis of these trials did not identify an improvement in adherence with EPR (89). As with APAP, the EPR algorithm varies by manufacturer and several manufacturers provide advances beyond pressure relief in expiration alone including the addition of inspiratory pressure relief in late inspiration for patients on BPAP. A small trial comparing APAP alone, APAP with EPR, and APAP with both inspiratory and EPR found the highest level of adherence among those randomized to APAP with EPR (90).
Adaptive servo-ventilation (ASV)
A further advancement in PAP technology has been the development of ASV as a mode of therapy. In ASV, BPAP is delivered but the level of ventilatory support (IPAP-EPAP) provided varies over time. The goal of ASV is to eliminate obstructive events with the EPAP and to hold ventilation at a fixed level by providing ventilatory support in inverse proportion to the patient’s own ventilation. As a result, periodic breathing (in the timeframe over which the ASV algorithm’s assessment of ventilation is being made) will be dampened out (91). ASV has shown high efficacy in resolving Cheyne-Stokes breathing associated with congestive heart failure alone or associated with OSA (92,93). However, a preliminary analysis of the Treatment of Predominant Central Sleep Apnoea by Adaptive Servo Ventilation in Patients with Heart Failure (SERVE-HF) phase III randomized trial, found an increased risk of cardiovascular mortality in those randomized to ASV (94,95). A more complete analysis of the results is pending to understand the cause of this unexpected finding. In the meantime, another randomized trial of ASV, the Effect of Adaptive Servo Ventilation (ASV) on Survival and Hospital Admissions in Heart Failure (ADVENT-HF), which includes those with OSA and those with milder levels of heart failure is ongoing (96). Some have advocated the use of ASV in the treatment of CPAP-emergent central apneas as well; however, a randomized trial of CPAP vs. ASV in CPAP-emergent central apneas found no benefit of ASV in terms of PAP adherence or symptoms. Thus, there is currently no strong rationale for the use of ASV in this setting (97).
CPAP modifications—humidification and mask choice
Early studies reported nasal dryness was noted in 44-65% of patients treated with nasal CPAP suggesting humidification may improve tolerance (98,99). Three randomized controlled trials have been performed evaluating the effect of adding heated humidification to the CPAP circuit. The first trial demonstrated a small improvement in adherence (100), but two larger subsequent trials found no benefit. Sleepiness has not been significantly affected by humidification (100-102). Nevertheless, given the possibility that heated humidification may improve compliance and satisfaction, it is now standard on all PAP machines available today, and can typically be turned off based on patient preference.
Given that as many as 50% of patients have at least one complaint regarding their mask interface (103), improving mask fit has been another target for increasing CPAP adherence. There are a wide range of mask types available today including nasal masks, oronasal masks, nasal pillow masks, hybrid oral masks with nasal pillows, and oral only masks (Figure 4). One small randomized trial found CPAP use was 1 hour per night greater with a nasal mask versus an oronasal mask (104). However, a subsequent meta-analysis found no consistent difference between mask types (105). Nevertheless, a large observational study found oronasal mask use was an independent predictor of non-adherence (76). Of note, the oronasal mask was typically prescribed as rescue therapy so these results may be confounded. Nevertheless, data demonstrating that pressure requirements may be higher with an oronasal mask suggest a nasal mask may be preferred as first choice (106,107). However, given the wide range of facial structures and nasal pathology, it is important to tailor mask choice to patient preferences.
Adjuvant hypnotic use
Because CPAP use may initially produce discomfort from the mask as well as anxiety, treatment of acute insomnia with short term use of a hypnotic drug has been advocated by some to reduce sleep latency and improve sleep continuity during the initiation of PAP therapy. A controlled clinical trial randomized OSA patients to 14 days of eszopiclone or placebo upon initiation of CPAP therapy. After 6 months, adherence was significantly better in the eszopiclone group by approximately 1 hour/night (108). In contrast, a similarly conducted trial using zolpidem found no benefit of hypnotic use (109). Another trial evaluating the effect of one night of zaleplon vs. placebo prior to a split night diagnostic polysomnogram/CPAP titration also found no difference in CPAP adherence 1 month later (110).
Education
Because reduced understanding of disease and treatment has been associated with lower levels of adherence in many chronic diseases, educational interventions to improve patient understanding of OSA and the benefits of CPAP have been developed. Several trials have been conducted to test the effect of intensive education regarding OSA and CPAP on patient compliance. Interventions that have been assessed include intensive education by practitioners and homecare providers (111) as well as use of standardized audiovisual presentations and demonstrations (112,113). To date, none of the educational interventions has consistently improved CPAP compliance. Many of these trials have been criticized in that the level of education provided to the control arm exceeds what is typically provided in routine care. As a result, most experts still recommend a basic level of education to all patients initiating CPAP therapy (114,115).
Motivational interviewing and cognitive behavioral therapy (CBT)
Psychosocial interventions with motivational interviewing and CBT have shown promise in augmenting CPAP compliance. Motivational interviewing is a type of psychosocial intervention in which a therapist elicits and targets an individual’s behavior by assessing the subject’s readiness to change, perceived importance of the change, and confidence in their ability to change. This motivational style has proven useful in smoking cessation (116) and the management of other chronic diseases (117). A three-arm randomized controlled trial comparing standard care, education, and motivational interviewing in CPAP naïve individuals demonstrated a trend towards increased adherence with the use of two 45-minute motivational interviewing sessions compared to standard care (118). A subsequent larger trial, however, did not identify a benefit at 12 months (119). Another trial using an intervention which involved three 30-minute motivational interviewing sessions over the first month of therapy demonstrated approximately 1.5 hours/night improvement in CPAP use at 3 months; however, no differences remained at 1 year (120). More recently, a Chinese trial randomized patients to either standard care or a pathway of interventions including educational and motivational visits and phone calls over 3 months tailored to their readiness to accept CPAP. The intervention group had greater CPAP adherence at 1 and 3 months (121). A less intensive strategy involving just one 20-minute interview and a 10-minute follow-up call using motivational interviewing was also successful in improving CPAP usage (122).
CBT, a psychosocial intervention aimed at correcting irrational and incorrect beliefs in order to alter behavior, has also been used to augment CPAP adherence. A randomized controlled trial evaluated the impact of two 1-hour group sessions (ten participants per group) compared to standard care and found those randomized to CBT used CPAP 2.9 hours longer than controls (123). These results indicate that behavioral therapy can have a large impact on CPAP adherence. However, subsequent research using a lower dose of CBT has not demonstrated a significant benefit, calling the feasibility of CBT as routine care into question (124).
Peer and spousal support
In addition to support from clinicians, the effect of peer support in improving CPAP adherence has also been studied. In a cohort of military veterans, “peer buddies” (fellow OSA patients with good CPAP compliance) met with newly diagnosed patients twice to provide support. This intervention led to improved compliance compared to control (125).
A major source of support for patients can also be their bed-partners. In observational studies, the presence of a bed-partner is associated with greater CPAP adherence (126). The presence of a bed-partner can also have negative consequences. Concerns about sexuality and bed-partner disturbance can adversely affect CPAP use (127,128), and perception of spousal “pressure” to use CPAP is associated with worse adherence (129,130). Nevertheless, in severe OSA, perceived spousal support correlates with greater CPAP use (130), and a perceived collaborative relationship with one’s spouse also predicts better adherence (131). Thus far, no studies have evaluated the impact of interventions aimed at improving bed-partner support.
Telemedicine
With the advent of the digital age, the application of telecommunication devices to ease communication between patient and provider has been applied to a number of chronic diseases (132-135). Unsurprisingly, a number of studies have been performed to evaluate the effect of telemedicine interventions on CPAP adherence. Using data wirelessly transmitted by patient’s CPAP units, investigators in one study monitored compliance on a daily basis. If a patient was found to have excessive mask leak, high AHI, or low duration of use, a research assistant would call the patient and troubleshoot problems. After 3 months, the group assigned to the telemedicine intervention had 1.88 hours/night greater CPAP use (136). A similarly designed randomized trial evaluated the effect of remote compliance surveillance by study staff in addition to a web-based service in which the patient could observe their own usage data. Patients randomized to the telemedicine arm had greater adherence at 2 months (137). A limitation of both studies is the cost in time and effort for an individual to survey the compliance data regularly for a large sample of patients. A lower cost alternative has also been studied. In a veteran’s population, patients were randomized to either usual care or a series of weekly automated telephone calls in which patients were able to report side effects of CPAP use and obtain pre-recorded advice. In addition, these reports were relayed to providers. At 1 year, patients in the intervention group demonstrated significantly higher adherence rates. Of note, however, mean usage was poor in both groups (138).
Alternative and adjunctive therapies to PAP
Because CPAP cannot be tolerated by all patients, there is a role for other treatments either as alternative therapies or as adjunctive treatments to reduce PAP requirements.
Weight loss
Given that obesity is one of the strongest risk factors for OSA, weight loss clearly improves OSA severity (139,140). Randomized trials in mild OSA suggest weight loss programs can resolve OSA but even in more severe disease, weight loss reduces OSA severity and improves symptoms (141,142). Furthermore, exercise itself may improve OSA even in the absence of weight loss (143). Bariatric surgery has increasingly been advocated in the treatment of OSA. A recent trial comparing gastric banding with intensive medical weight loss as adjunctive treatments to CPAP, found at 2 years that the AHI had reduced by 31.6% in the surgical arm although this change was not significantly different from the medical arm. However, few patients in either arm were able to stop CPAP (144). Another trial assessing the impact of medical weight loss as adjunctive therapy to CPAP found weight loss tended to have a more beneficial impact on cardiovascular markers than CPAP and the greatest improvements were in those receiving both CPAP and weight loss (43). The importance of combining weight loss therapy with CPAP is further emphasized by emerging data demonstrating CPAP treatment leads to weight gain (17).
Sleep position
Due to the effects of gravity on upper airway anatomy, OSA severity is often worse in the supine position, and a substantial number of patients only have airway collapse while supine (145,146). In these patients, positional therapies can improve sleep apnea as monotherapy although long term compliance—even when assistive devices are employed—is only about 25% (147). In theory, combining CPAP with positional therapy may allow for the use of lower CPAP levels. A post-hoc analysis found the greatest benefit of APAP vs. CPAP was in those patients with highly positional disease, where presumably the APAP could use substantially lower pressures when patients were non-supine (148).
Mandibular advancement devices (MADs)
MADs have become firmly rooted as second line therapy in the treatment of OSA. Randomized trials have demonstrated the efficacy of MADs to reduce sleepiness and blood pressure in OSA (149,150). While CPAP improves the AHI to a greater extent than MAD therapy (149,151-153), patient satisfaction may be greater with MADs (154). A recent comparative effectiveness trial found no difference between CPAP and MAD in treating sleepiness due to greater improvement of OSA with CPAP but greater adherence with MAD (155). Of note, neither treatment improved blood pressure in this study. One small trial has assessed the impact of adding MAD to CPAP. In patients previously intolerant to CPAP, MAD reduced the applied pressure required, frequency of residual apneas, and residual sleepiness (156).
Surgery
The most commonly performed surgery to treat OSA is the uvulopalatopharyngoplasty (UPPP) which involves a tonsillectomy, excision of the uvula and posterior palate and trimming of the posterior pillars. This procedure lowers the AHI below 10/h in only 33% of patients (157). Furthermore, UPPP may compromise future CPAP use, as patients with UPPP are more likely to be non-compliant, have increased air leak, and lower pressure tolerance than matched controls (158). Maxillo-mandibular advancement (MMA) is a procedure which advances the anterior pharyngeal palate and enlarges the mandible. In uncontrolled studies, MMA is highly efficacious, resulting in a mean residual AHI of 7.7/hour (159). However, given the morbidity of the surgery, MMA is infrequently utilized (160). Tracheostomy cures OSA by bypassing the upper airway completely but is also infrequently utilized due to the high associated morbidity including recurrent pneumonia, stomal complications, and psychological trauma (161).
Oral pressure therapy (OPT)
OPT is a novel treatment option which involves the application of negative pressure in the mouth to pull the tongue anteriorly. A small, unblinded trial demonstrated a statistically significant reduction in AHI and an improvement in subjective sleepiness with OPT. Compliance with the device, 6.0±1.4 hour, was higher than commonly seen with PAP (162). More definitive studies are needed to understand the role of OPT in OSA management.
Expiratory positive airway pressure (EPAP)
The application of EPAP at the nose through the use of one-way valves has also been developed as an alternative to CPAP. The valves freely allow inspiration, but inhibit expiration until a sufficient amount of expiratory pressure is generated. An initial randomized trial demonstrated improvements in AHI and sleepiness with EPAP therapy (163). However, a subsequent larger trial in prior CPAP users found a high failure rate with EPAP, not significantly different from placebo (164).
Hypoglossal nerve stimulation (HGNS)
Given the role of the upper airway dilator muscles in maintaining airway patency (165), stimulation of the hypoglossal nerve to increase upper airway muscle tone has emerged as a potential therapy for OSA. HGNS involves surgical placement of a pacing electrode on the hypoglossal nerve along with a sensing electrode on an intercostal muscle to synchronize pacing to inspiration. Preliminary uncontrolled studies demonstrated that HGNS was feasible in improving OSA severity, sleepiness, and quality of life (166,167), but certain groups (those with concentric palatal collapse or morbid obesity) were less likely to respond. Several HGNS systems have been developed. One of these devices was found to be ineffective (168) but a second device produced significant improvements in AHI and sleepiness in a highly selected population (169).
Wake-promoting agents
Residual sleepiness is common even in CPAP compliant OSA patients. Among those using CPAP >6 hours/night, subjective sleepiness is noted in 22% and objective sleepiness in 52% (170). The wake-promoting agent, modafinil, when added to CPAP, improves both subjective and objective measures of sleepiness with a mean effect on ESS of 6-7 points. Although there is concern that modafinil use may lead to reductions in CPAP adherence, this effect has not been consistently observed in clinical trials (171-174). Modafinil is also effective as adjunctive therapy when CPAP is stopped for short periods of time (e.g., when traveling), preventing re-emergence of sleepiness (175). In general, armodafinil produces similar results as modafinil (176,177).
Individualized treatment of disease
The traditional model of OSA pathophysiology is that of compromised upper airway anatomy leading to repetitive collapse during sleep (178,179). However, our understanding of the pathophysiology of OSA has broadened in recent years to include traits beyond compromised anatomy (180,181). At least three further physiological factors interact to play a role in OSA including (I) inadequate upper airway dilator muscle responsiveness (180,182); (II) an abnormal respiratory arousal threshold (183,184); and (III) a hypersensitive ventilatory control system (elevated loop gain) (185). The increasing recognition of the importance of these traits has led to the idea of an individualized approach to therapy, where the specific underlying mechanisms in each patient are addressed via novel focused therapies.
The upper airway dilator muscles play an important role in maintaining airway patency during both sleep and wakefulness (186). The largest of these muscles is the genioglossus, which is innervated by the hypoglossal nerve (187-190). Contraction of this muscle appears to be both necessary and sufficient to prevent pharyngeal collapse in patients with OSA (165). However, responsiveness of the genioglossus to respiratory stimuli such as negative pressure and hypercapnia during sleep is variable, and overall there is decreased descending neural stimulation to the dilator muscles at sleep onset (191). Ultimately some individuals demonstrate a robust response leading to maintenance of airway patency, and others an insufficient response (192-194).
Interestingly, awake OSA patients have higher electromyographic signals in the genioglossus compared to healthy controls, suggesting a compensatory response to airway vulnerability (195). The loss of this heightened neuromuscular stimulation at sleep onset is theorized to contribute to airway collapse in susceptible individuals (196). A particular subgroup of OSA patients has insufficient genioglossus responsiveness, perhaps suggesting an important role of this trait in apnea pathogenesis as well as providing a potential therapeutic target (197). Current understanding of the pathways leading to genioglossal activation is limited making targeted pharmacologic intervention difficult, though studies of tricyclic antidepressants (196,198), ampakines (199), and cholinesterase inhibitors (200) are ongoing. Other pharmacologic interventions are focused on increasing the arousal threshold (discussed below), which may allow for successive dilator muscle recruitment and subsequent airway opening prior to arousal. Finally, electrical stimulation of the hypoglossal nerve or the dilator muscles directly, as previously discussed, may be a viable intervention in some cases (201-204).
Another physiological trait of interest in OSA is the arousal threshold. When a stimulus increases to a level of intensity that it causes awakening from sleep, the arousal threshold has been reached. An individual with a low arousal threshold is awoken easily, and an individual with a high arousal threshold is relatively resistant to awakening. In regard to OSA, the major stimulus to arousal includes intrathoracic pressure which is a function of respiratory efforts during hypoxemia and hypercapnia (205). Arousal is believed to be an important defense mechanism to protect against severe hypoxemia, and an arousal threshold that is too high could contribute to tissue hypoxia during apnea (206). Conversely a low arousal threshold in the setting of OSA leads to repetitive awakenings prior to sufficient recruitment of airway dilator muscles (207,208). Each such awakening is associated with an acute ventilatory escalation due in part to hypercapnia and a concurrent drop in CO2 setpoint (209,210). Recurrent surges in ventilation can in turn contribute to respiratory instability and further episodes of apnea and hypopnea, most dramatically in patients with elevated loop gain (described below) (208,211).
Although patients with OSA on average have a somewhat higher arousal threshold in comparison to controls, a low arousal threshold likely plays a role in disease pathophysiology in roughly one third of affected patients (183,197,207). For these patients an increase in arousal threshold, for example via pharmacologic intervention, may allow for recruitment of airway dilator muscles and avoidance of respiratory instability (208). Concerns related to such therapies include the possibility of blunting arousal in response to severe hypoxia as well as suppressing upper airway dilator muscle activity, thereby worsening apnea (212). The hypnotic trazodone, has been shown to increase arousal threshold to CO2 in patients with OSA and a low arousal threshold, without impairing pharyngeal muscle activity or increasing airway collapsibility (206,212,213). Other non-myorelaxant sedatives such as triazolam and eszopiclone have demonstrated a similar effect on arousal threshold (214,215).
The respiratory control system is a loop made up of the lungs, circulating blood, chemoreceptors, and descending neurologic signals that manage ventilatory drive. The sensitivity of this system to changes in respiratory stimuli can be conceptualized using the engineering notion of loop gain, which represents the stability of a negative feedback loop (216). In relation to respiratory control, loop gain is defined as the ratio of a ventilatory response over the perturbation in ventilation. In a system with high loop gain, a small change in respiratory stimuli (e.g., hypercapnia) leads to an exaggerated ventilatory response. This overcorrection can lead to respiratory instability (self-sustaining oscillations). In contrast, a system with low loop gain is likely to maintain respiratory stability (185,217). There are three major components that affect loop gain. The first is ‘plant gain’, or changes in the respiratory apparatus including the lungs and circulating blood in response to neuronal stimulation. The second is ‘controller gain’, or the sensitivity of chemoreceptors to oxygen and CO2 levels and the subsequent output from respiratory centers eliciting a response from the plant. The third is the circulation delay between the plant (lungs) and controller (chemoreceptors), which leads to a lag in response to changes in blood gases.
Not all OSA patients have an abnormal loop gain. However, in a subgroup of individuals with OSA and only mild airway collapsibility, loop gain was nearly 50% greater in comparison to controls (197). This finding suggests that loop gain may play a role in OSA pathophysiology in a subset of patients, and offers a possible therapeutic target. Of the three components, only controller gain has been shown to be elevated in OSA patients (185,217,218). Several therapies impacting loop gain are being evaluated as potential treatments for OSA. Supplemental oxygen decreases loop gain in patients with high baseline loop gain via reduction in controller gain, without significantly affecting pharyngeal collapsibility, upper airway responsiveness, or arousal threshold (219,220). In contrast, the carbonic anhydrase inhibitor acetazolamide lowers loop gain (regardless of baseline levels) primarily via reduction in plant gain, likely due to a lowering of PaCO2 (221).
Conclusions
The most effective and reliable treatment for OSA today remains CPAP, whose main limitation is tolerability resulting in suboptimal patient adherence. The coming decades will demand new interventions to augment CPAP adherence and alternative therapies tailored to the individual patient. Even without newer options, further research is required to individualize treatment options optimally.
Acknowledgements
We would like to acknowledge Dr. Stephen H. Loring, MD, Robert Chase RRT and Dr. Thomas E. Scammell, MD, for assistance with figures.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Strohl KP, Redline S. Recognition of obstructive sleep apnea. Am J Respir Crit Care Med 1996;154:279-89. [PubMed]
- Peppard PE, Young T, Barnet JH, et al. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol 2013;177:1006-14. [PubMed]
- Sullivan CE, Issa FG, Berthon-Jones M, et al. Reversal of obstructive sleep apnoea by continuous positive airway pressure applied through the nares. Lancet 1981;1:862-5. [PubMed]
- Epstein LJ, Kristo D, Strollo PJ Jr, et al. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med 2009;5:263-76. [PubMed]
- Schwab RJ, Pack AI, Gupta KB, et al. Upper airway and soft tissue structural changes induced by CPAP in normal subjects. Am J Respir Crit Care Med 1996;154:1106-16. [PubMed]
- Pasterkamp H, Schäfer J, Wodicka GR. Posture-dependent change of tracheal sounds at standardized flows in patients with obstructive sleep apnea. Chest 1996;110:1493-8. [PubMed]
- Li D, Liu D, Wang X, et al. Self-reported habitual snoring and risk of cardiovascular disease and all-cause mortality. Atherosclerosis 2014;235:189-95. [PubMed]
- Berry RB, Block AJ. Positive nasal airway pressure eliminates snoring as well as obstructive sleep apnea. Chest 1984;85:15-20. [PubMed]
- Beninati W, Harris CD, Herold DL, et al. The effect of snoring and obstructive sleep apnea on the sleep quality of bed partners. Mayo Clin Proc 1999;74:955-8. [PubMed]
- Cruz IA, Drummond M, Winck JC. Obstructive sleep apnea symptoms beyond sleepiness and snoring: effects of nasal APAP therapy. Sleep Breath 2012;16:361-6. [PubMed]
- Seneviratne U, Puvanendran K. Excessive daytime sleepiness in obstructive sleep apnea: prevalence, severity, and predictors. Sleep Med 2004;5:339-43. [PubMed]
- Goncalves MA, Paiva T, Ramos E, et al. Obstructive sleep apnea syndrome, sleepiness, and quality of life. Chest 2004;125:2091-6. [PubMed]
- Giles TL, Lasserson TJ, Smith BJ, et al. Continuous positive airways pressure for obstructive sleep apnoea in adults. Cochrane Database Syst Rev 2006.CD001106. [PubMed]
- Weaver TE, Mancini C, Maislin G, et al. Continuous positive airway pressure treatment of sleepy patients with milder obstructive sleep apnea: results of the CPAP Apnea Trial North American Program (CATNAP) randomized clinical trial. Am J Respir Crit Care Med 2012;186:677-83. [PubMed]
- Orth M, Duchna HW, Leidag M, et al. Driving simulator and neuropsychological [corrected] testing in OSAS before and under CPAP therapy. Eur Respir J 2005;26:898-903. [PubMed]
- Tregear S, Reston J, Schoelles K, et al. Continuous positive airway pressure reduces risk of motor vehicle crash among drivers with obstructive sleep apnea: systematic review and meta-analysis. Sleep 2010;33:1373-80. [PubMed]
- Kushida CA, Nichols DA, Holmes TH, et al. Effects of continuous positive airway pressure on neurocognitive function in obstructive sleep apnea patients: The Apnea Positive Pressure Long-term Efficacy Study (APPLES). Sleep 2012;35:1593-602. [PubMed]
- Cooke JR, Ayalon L, Palmer BW, et al. Sustained use of CPAP slows deterioration of cognition, sleep, and mood in patients with Alzheimer's disease and obstructive sleep apnea: a preliminary study. J Clin Sleep Med 2009;5:305-9. [PubMed]
- Ancoli-Israel S, Palmer BW, Cooke JR, et al. Cognitive effects of treating obstructive sleep apnea in Alzheimer's disease: a randomized controlled study. J Am Geriatr Soc 2008;56:2076-81. [PubMed]
- Chakravorty I, Cayton RM, Szczepura A. Health utilities in evaluating intervention in the sleep apnoea/hypopnoea syndrome. Eur Respir J 2002;20:1233-8. [PubMed]
- Monasterio C, Vidal S, Duran J, et al. Effectiveness of continuous positive airway pressure in mild sleep apnea-hypopnea syndrome. Am J Respir Crit Care Med 2001;164:939-43. [PubMed]
- Barbé F, Durán-Cantolla J, Sánchez-de-la-Torre M, et al. Effect of continuous positive airway pressure on the incidence of hypertension and cardiovascular events in nonsleepy patients with obstructive sleep apnea: a randomized controlled trial. JAMA 2012;307:2161-8. [PubMed]
- Buchner NJ, Sanner BM, Borgel J, et al. Continuous positive airway pressure treatment of mild to moderate obstructive sleep apnea reduces cardiovascular risk. Am J Respir Crit Care Med 2007;176:1274-80. [PubMed]
- Chaudhary BA, Elguindi AS, King DW. Obstructive sleep apnea after lateral medullary syndrome. South Med J 1982;75:65-7. [PubMed]
- Gottlieb DJ, Yenokyan G, Newman AB, et al. Prospective study of obstructive sleep apnea and incident coronary heart disease and heart failure: the sleep heart health study. Circulation 2010;122:352-60. [PubMed]
- Kiely JL, McNicholas WT. Cardiovascular risk factors in patients with obstructive sleep apnoea syndrome. Eur Respir J 2000;16:128-33. [PubMed]
- Marin JM, Carrizo SJ, Vicente E, et al. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet 2005;365:1046-53. [PubMed]
- Oldenburg O, Lamp B, Faber L, et al. Sleep-disordered breathing in patients with symptomatic heart failure: a contemporary study of prevalence in and characteristics of 700 patients. Eur J Heart Fail 2007;9:251-7. [PubMed]
- Redline S, Yenokyan G, Gottlieb DJ, et al. Obstructive sleep apnea-hypopnea and incident stroke: the sleep heart health study. Am J Respir Crit Care Med 2010;182:269-77. [PubMed]
- Sin DD, Fitzgerald F, Parker JD, et al. Risk factors for central and obstructive sleep apnea in 450 men and women with congestive heart failure. Am J Respir Crit Care Med 1999;160:1101-6. [PubMed]
- Peppard PE, Young T, Palta M, et al. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med 2000;342:1378-84. [PubMed]
- Marin JM, Agusti A, Villar I, et al. Association between treated and untreated obstructive sleep apnea and risk of hypertension. JAMA 2012;307:2169-76. [PubMed]
- Bazzano LA, Khan Z, Reynolds K, et al. Effect of nocturnal nasal continuous positive airway pressure on blood pressure in obstructive sleep apnea. Hypertension 2007;50:417-23. [PubMed]
- Barbé F, Mayoralas LR, Duran J, et al. Treatment with continuous positive airway pressure is not effective in patients with sleep apnea but no daytime sleepiness. a randomized, controlled trial. Ann Intern Med 2001;134:1015-23. [PubMed]
- Craig SE, Kohler M, Nicoll D, et al. Continuous positive airway pressure improves sleepiness but not calculated vascular risk in patients with minimally symptomatic obstructive sleep apnoea: the MOSAIC randomised controlled trial. Thorax 2012;67:1090-6. [PubMed]
- Robinson GV, Smith DM, Langford BA, et al. Continuous positive airway pressure does not reduce blood pressure in nonsleepy hypertensive OSA patients. Eur Respir J 2006;27:1229-35. [PubMed]
- Bratton DJ, Stradling JR, Barbé F, et al. Effect of CPAP on blood pressure in patients with minimally symptomatic obstructive sleep apnoea: a meta-analysis using individual patient data from four randomised controlled trials. Thorax 2014;69:1128-35. [PubMed]
- Pedrosa RP, Drager LF, de Paula LK, et al. Effects of OSA treatment on BP in patients with resistant hypertension: a randomized trial. Chest 2013;144:1487-94. [PubMed]
- Martínez-García MA, Capote F, Campos-Rodríguez F, et al. Effect of CPAP on blood pressure in patients with obstructive sleep apnea and resistant hypertension: the HIPARCO randomized clinical trial. JAMA 2013;310:2407-15. [PubMed]
- Punjabi NM, Shahar E, Redline S, et al. Sleep-disordered breathing, glucose intolerance, and insulin resistance: the Sleep Heart Health Study. Am J Epidemiol 2004;160:521-30. [PubMed]
- Nadeem R, Singh M, Nida M, et al. Effect of obstructive sleep apnea hypopnea syndrome on lipid profile: a meta-regression analysis. J Clin Sleep Med 2014;10:475-89. [PubMed]
- Stamatakis K, Sanders MH, Caffo B, et al. Fasting glycemia in sleep disordered breathing: lowering the threshold on oxyhemoglobin desaturation. Sleep 2008;31:1018-24. [PubMed]
- Chirinos JA, Gurubhagavatula I, Teff K, et al. CPAP, weight loss, or both for obstructive sleep apnea. N Engl J Med 2014;370:2265-75. [PubMed]
- Dorkova Z, Petrasova D, Molcanyiova A, et al. Effects of continuous positive airway pressure on cardiovascular risk profile in patients with severe obstructive sleep apnea and metabolic syndrome. Chest 2008;134:686-92. [PubMed]
- Hoyos CM, Killick R, Yee BJ, et al. Cardiometabolic changes after continuous positive airway pressure for obstructive sleep apnoea: a randomised sham-controlled study. Thorax 2012;67:1081-9. [PubMed]
- Weinstock TG, Wang X, Rueschman M, et al. A controlled trial of CPAP therapy on metabolic control in individuals with impaired glucose tolerance and sleep apnea. Sleep 2012;35:617-625B. [PubMed]
- Myhill PC, Davis WA, Peters KE, et al. Effect of continuous positive airway pressure therapy on cardiovascular risk factors in patients with type 2 diabetes and obstructive sleep apnea. J Clin Endocrinol Metab 2012;97:4212-8. [PubMed]
- Keenan BT, Maislin G, Sunwoo BY, et al. Obstructive sleep apnoea treatment and fasting lipids: a comparative effectiveness study. Eur Respir J 2014;44:405-14. [PubMed]
- Kohler M, Stoewhas AC, Ayers L, et al. Effects of continuous positive airway pressure therapy withdrawal in patients with obstructive sleep apnea: a randomized controlled trial. Am J Respir Crit Care Med 2011;184:1192-9. [PubMed]
- Phillips CL, Yee BJ, Marshall NS, et al. Continuous positive airway pressure reduces postprandial lipidemia in obstructive sleep apnea: a randomized, placebo-controlled crossover trial. Am J Respir Crit Care Med 2011;184:355-61. [PubMed]
- Sivam S, Phillips CL, Trenell MI, et al. Effects of 8 weeks of continuous positive airway pressure on abdominal adiposity in obstructive sleep apnoea. Eur Respir J 2012;40:913-8. [PubMed]
- Kritikou I, Basta M, Tappouni R, et al. Sleep apnoea and visceral adiposity in middle-aged male and female subjects. Eur Respir J 2013;41:601-9. [PubMed]
- Redenius R, Murphy C, O'Neill E, et al. Does CPAP lead to change in BMI? J Clin Sleep Med 2008;4:205-9. [PubMed]
- Bourey R, Bourey J, Habbal N, et al. Early gain in body mass with CPAP therapy for obstructive sleep apnea. Somnologie 2010;14:207-12.
- Quan SF, Budhiraja R, Clarke DP, et al. Impact of treatment with continuous positive airway pressure (CPAP) on weight in obstructive sleep apnea. J Clin Sleep Med 2013;9:989-93. [PubMed]
- Phillips B, Dhaon NA. Weigh the options before starting CPAP. J Clin Sleep Med 2013;9:995-6. [PubMed]
- Parra O, Sánchez-Armengol A, Bonnin M, et al. Early treatment of obstructive apnoea and stroke outcome: a randomised controlled trial. Eur Respir J 2011;37:1128-36. [PubMed]
- Ryan CM, Bayley M, Green R, et al. Influence of continuous positive airway pressure on outcomes of rehabilitation in stroke patients with obstructive sleep apnea. Stroke 2011;42:1062-7. [PubMed]
- Esquinas C, Sánchez-de-la Torre M, Aldomá A, et al. Rationale and methodology of the impact of continuous positive airway pressure on patients with ACS and nonsleepy OSA: the ISAACC Trial. Clin Cardiol 2013;36:495-501. [PubMed]
- Peker Y, Glantz H, Thunström E, et al. Rationale and design of the Randomized Intervention with CPAP in Coronary Artery Disease and Sleep Apnoea--RICCADSA trial. Scand Cardiovasc J 2009;43:24-31. [PubMed]
- Kaneko Y, Floras JS, Usui K, et al. Cardiovascular effects of continuous positive airway pressure in patients with heart failure and obstructive sleep apnea. N Engl J Med 2003;348:1233-41. [PubMed]
- Mansfield DR, Gollogly NC, Kaye DM, et al. Controlled trial of continuous positive airway pressure in obstructive sleep apnea and heart failure. Am J Respir Crit Care Med 2004;169:361-6. [PubMed]
- Arias MA, García-Río F, Alonso-Fernández A, et al. Obstructive sleep apnea syndrome affects left ventricular diastolic function: effects of nasal continuous positive airway pressure in men. Circulation 2005;112:375-83. [PubMed]
- Damy T, Margarit L, Noroc A, et al. Prognostic impact of sleep-disordered breathing and its treatment with nocturnal ventilation for chronic heart failure. Eur J Heart Fail 2012;14:1009-19. [PubMed]
- Kasai T, Narui K, Dohi T, et al. Prognosis of patients with heart failure and obstructive sleep apnea treated with continuous positive airway pressure. Chest 2008;133:690-6. [PubMed]
- Arias MA, García-Río F, Alonso-Fernández A, et al. Pulmonary hypertension in obstructive sleep apnoea: effects of continuous positive airway pressure: a randomized, controlled cross-over study. Eur Heart J 2006;27:1106-13. [PubMed]
- Fein AS, Shvilkin A, Shah D, et al. Treatment of obstructive sleep apnea reduces the risk of atrial fibrillation recurrence after catheter ablation. J Am Coll Cardiol 2013;62:300-5. [PubMed]
- Kanagala R, Murali NS, Friedman PA, et al. Obstructive sleep apnea and the recurrence of atrial fibrillation. Circulation 2003;107:2589-94. [PubMed]
- Naruse Y, Tada H, Satoh M, et al. Concomitant obstructive sleep apnea increases the recurrence of atrial fibrillation following radiofrequency catheter ablation of atrial fibrillation: clinical impact of continuous positive airway pressure therapy. Heart Rhythm 2013;10:331-7. [PubMed]
- Campos-Rodriguez F, Martinez-Garcia MA, Martinez M, et al. Association between obstructive sleep apnea and cancer incidence in a large multicenter Spanish cohort. Am J Respir Crit Care Med 2013;187:99-105. [PubMed]
- Nieto FJ, Peppard PE, Young T, et al. Sleep-disordered breathing and cancer mortality: results from the Wisconsin Sleep Cohort Study. Am J Respir Crit Care Med 2012;186:190-4. [PubMed]
- Martínez-García MA, Campos-Rodríguez F, Catalán-Serra P, et al. Cardiovascular mortality in obstructive sleep apnea in the elderly: role of long-term continuous positive airway pressure treatment: a prospective observational study. Am J Respir Crit Care Med 2012;186:909-16. [PubMed]
- Marti S, Sampol G, Muñoz X, et al. Mortality in severe sleep apnoea/hypopnoea syndrome patients: impact of treatment. Eur Respir J 2002;20:1511-8. [PubMed]
- Wozniak DR, Lasserson TJ, Smith I. Educational, supportive and behavioural interventions to improve usage of continuous positive airway pressure machines in adults with obstructive sleep apnoea. Cochrane Database Syst Rev 2014;1:CD007736. [PubMed]
- Schwab RJ, Badr SM, Epstein LJ, et al. An official American Thoracic Society statement: continuous positive airway pressure adherence tracking systems. The optimal monitoring strategies and outcome measures in adults. Am J Respir Crit Care Med 2013;188:613-20. [PubMed]
- Borel JC, Tamisier R, Dias-Domingos S, et al. Type of mask may impact on continuous positive airway pressure adherence in apneic patients. PLoS One 2013;8:e64382. [PubMed]
- Kribbs NB, Pack AI, Kline LR, et al. Objective measurement of patterns of nasal CPAP use by patients with obstructive sleep apnea. Am Rev Respir Dis 1993;147:887-95. [PubMed]
- Hudgel DW, Harasick T. Fluctuation in timing of upper airway and chest wall inspiratory muscle activity in obstructive sleep apnea. J Appl Physiol (1985) 1990;69:443-50. [PubMed]
- Sanders MH, Kern N. Obstructive sleep apnea treated by independently adjusted inspiratory and expiratory positive airway pressures via nasal mask. Physiologic and clinical implications. Chest 1990;98:317-24. [PubMed]
- Atwood CW Jr. Progress toward a clearer understanding of the role of bilevel positive airway pressure therapy for obstructive sleep apnea. J Clin Sleep Med 2013;9:337-8. [PubMed]
- Schwartz SW, Rosas J, Iannacone MR, et al. Correlates of a prescription for Bilevel positive airway pressure for treatment of obstructive sleep apnea among veterans. J Clin Sleep Med 2013;9:327-35. [PubMed]
- Teschler H, Berthon-Jones M, Thompson AB, et al. Automated continuous positive airway pressure titration for obstructive sleep apnea syndrome. Am J Respir Crit Care Med 1996;154:734-40. [PubMed]
- Kakkar RK, Berry RB. Positive airway pressure treatment for obstructive sleep apnea. Chest 2007;132:1057-72. [PubMed]
- Ayas NT, Patel SR, Malhotra A, et al. Auto-titrating versus standard continuous positive airway pressure for the treatment of obstructive sleep apnea: results of a meta-analysis. Sleep 2004;27:249-53. [PubMed]
- Ip S, D'Ambrosio C, Patel K, et al. Auto-titrating versus fixed continuous positive airway pressure for the treatment of obstructive sleep apnea: a systematic review with meta-analyses. Syst Rev 2012;1:20. [PubMed]
- Planès C, D'Ortho MP, Foucher A, et al. Efficacy and cost of home-initiated auto-nCPAP versus conventional nCPAP. Sleep 2003;26:156-60. [PubMed]
- Powell ED, Gay PC, Ojile JM, et al. A pilot study assessing adherence to auto-bilevel following a poor initial encounter with CPAP. J Clin Sleep Med 2012;8:43-7. [PubMed]
- Aloia MS, Stanchina M, Arnedt JT, et al. Treatment adherence and outcomes in flexible vs standard continuous positive airway pressure therapy. Chest 2005;127:2085-93. [PubMed]
- Smith I, Lasserson TJ. Pressure modification for improving usage of continuous positive airway pressure machines in adults with obstructive sleep apnoea. Cochrane Database Syst Rev 2009.CD003531. [PubMed]
- Chihara Y, Tsuboi T, Hitomi T, et al. Flexible positive airway pressure improves treatment adherence compared with auto-adjusting PAP. Sleep 2013;36:229-36. [PubMed]
- Javaheri S, Brown LK, Randerath WJ. Positive airway pressure therapy with adaptive servoventilation: part 1: operational algorithms. Chest 2014;146:514-23. [PubMed]
- Birner C, Series F, Lewis K, et al. Effects of auto-servo ventilation on patients with sleep-disordered breathing, stable systolic heart failure and concomitant diastolic dysfunction: subanalysis of a randomized controlled trial. Respiration 2014;87:54-62. [PubMed]
- Kasai T, Kasagi S, Maeno K, et al. Adaptive servo-ventilation in cardiac function and neurohormonal status in patients with heart failure and central sleep apnea nonresponsive to continuous positive airway pressure. JACC Heart Fail 2013;1:58-63. [PubMed]
- ResMed. ResMed Provides Update on Phase IV SERVE-HF Study of Adaptive Servo-Ventilation (ASV) Therapy In Central Sleep Apnea and Chronic Heart Failure. Available online: http://www.resmed.com/us/en/consumer/newsandinformation/news-releases/2015/resmed-provides-update-on-phase-iv-serve-hf-study-of-adaptive-servo-ventilation-therapy.html
- Cowie MR, Woehrle H, Wegscheider K, et al. Rationale and design of the SERVE-HF study: treatment of sleep-disordered breathing with predominant central sleep apnoea with adaptive servo-ventilation in patients with chronic heart failure. Eur J Heart Fail 2013;15:937-43. [PubMed]
- Bradley D. Effect of Adaptive Servo Ventilation (ASV) on Survival and Hospital Admissions in Heart Failure (ADVENT-HF): Study Record Data. Available online: https://clinicaltrials.gov/ct2/show/NCT01128816
- Morgenthaler TI, Kuzniar TJ, Wolfe LF, et al. The complex sleep apnea resolution study: a prospective randomized controlled trial of continuous positive airway pressure versus adaptive servoventilation therapy. Sleep 2014;37:927-34. [PubMed]
- Sanders MH, Gruendl CA, Rogers RM. Patient compliance with nasal CPAP therapy for sleep apnea. Chest 1986;90:330-3. [PubMed]
- Hoffstein V, Viner S, Mateika S, et al. Treatment of obstructive sleep apnea with nasal continuous positive airway pressure. Patient compliance, perception of benefits, and side effects. Am Rev Respir Dis 1992;145:841-5. [PubMed]
- Massie CA, Hart RW, Peralez K, et al. Effects of humidification on nasal symptoms and compliance in sleep apnea patients using continuous positive airway pressure. Chest 1999;116:403-8. [PubMed]
- Neill AM, Wai HS, Bannan SP, et al. Humidified nasal continuous positive airway pressure in obstructive sleep apnoea. Eur Respir J 2003;22:258-62. [PubMed]
- Mador MJ, Krauza M, Pervez A, et al. Effect of heated humidification on compliance and quality of life in patients with sleep apnea using nasal continuous positive airway pressure. Chest 2005;128:2151-8. [PubMed]
- Pépin JL, Leger P, Veale D, et al. Side effects of nasal continuous positive airway pressure in sleep apnea syndrome. Study of 193 patients in two French sleep centers. Chest 1995;107:375-81. [PubMed]
- Mortimore IL, Whittle AT, Douglas NJ. Comparison of nose and face mask CPAP therapy for sleep apnoea. Thorax 1998;53:290-2. [PubMed]
- Chai CL, Pathinathan A, Smith B. Continuous positive airway pressure delivery interfaces for obstructive sleep apnoea. Cochrane Database Syst Rev 2006.CD005308. [PubMed]
- Ebben MR, Narizhnaya M, Segal AZ, et al. A randomised controlled trial on the effect of mask choice on residual respiratory events with continuous positive airway pressure treatment. Sleep Med 2014;15:619-24. [PubMed]
- Kaminska M, Montpetit A, Mathieu A, et al. Higher effective oronasal versus nasal continuous positive airway pressure in obstructive sleep apnea: effect of mandibular stabilization. Can Respir J 2014;21:234-8. [PubMed]
- Lettieri CJ, Shah AA, Holley AB, et al. Effects of a short course of eszopiclone on continuous positive airway pressure adherence: a randomized trial. Ann Intern Med 2009;151:696-702. [PubMed]
- Bradshaw DA, Ruff GA, Murphy DP. An oral hypnotic medication does not improve continuous positive airway pressure compliance in men with obstructive sleep apnea. Chest 2006;130:1369-76. [PubMed]
- Park JG, Olson EJ, Morgenthaler TI. Impact of zaleplon on continuous positive airway pressure therapy compliance. J Clin Sleep Med 2013;9:439-44. [PubMed]
- Meurice JC, Ingrand P, Portier F, et al. A multicentre trial of education strategies at CPAP induction in the treatment of severe sleep apnoea-hypopnoea syndrome. Sleep Med 2007;8:37-42. [PubMed]
- Jean Wiese H, Boethel C, Phillips B, et al. CPAP compliance: video education may help! Sleep Med 2005;6:171-4. [PubMed]
- Golay A, Girard A, Grandin S, et al. A new educational program for patients suffering from sleep apnea syndrome. Patient Educ Couns 2006;60:220-7. [PubMed]
- Mehra R. Sleep apnea ABCs: airway, breathing, circulation. Cleve Clin J Med 2014;81:479-89. [PubMed]
- Wickwire EM, Lettieri CJ, Cairns AA, et al. Maximizing positive airway pressure adherence in adults: a common-sense approach. Chest 2013;144:680-93. [PubMed]
- Emmons KM, Hammond SK, Fava JL, et al. A randomized trial to reduce passive smoke exposure in low-income households with young children. Pediatrics 2001;108:18-24. [PubMed]
- Chlebowy DO, El-Mallakh P, Myers J, et al. Motivational interviewing to improve diabetes outcomes in African Americans adults with diabetes. West J Nurs Res 2015;37:566-80. [PubMed]
- Aloia MS, Smith K, Arnedt JT, et al. Brief behavioral therapies reduce early positive airway pressure discontinuation rates in sleep apnea syndrome: preliminary findings. Behav Sleep Med 2007;5:89-104. [PubMed]
- Aloia MS, Arnedt JT, Strand M, et al. Motivational enhancement to improve adherence to positive airway pressure in patients with obstructive sleep apnea: a randomized controlled trial. Sleep 2013;36:1655-62. [PubMed]
- Olsen S, Smith SS, Oei TP, et al. Motivational interviewing (MINT) improves continuous positive airway pressure (CPAP) acceptance and adherence: a randomized controlled trial. J Consult Clin Psychol 2012;80:151-63. [PubMed]
- Deng T, Wang Y, Sun M, et al. Stage-matched intervention for adherence to CPAP in patients with obstructive sleep apnea: a randomized controlled trial. Sleep Breath 2013;17:791-801. [PubMed]
- Lai AY, Fong DY, Lam JC, et al. The efficacy of a brief motivational enhancement education program on CPAP adherence in OSA: a randomized controlled trial. Chest 2014;146:600-10. [PubMed]
- Richards D, Bartlett DJ, Wong K, et al. Increased adherence to CPAP with a group cognitive behavioral treatment intervention: a randomized trial. Sleep 2007;30:635-40. [PubMed]
- Bartlett D, Wong K, Richards D, et al. Increasing adherence to obstructive sleep apnea treatment with a group social cognitive therapy treatment intervention: a randomized trial. Sleep 2013;36:1647-54. [PubMed]
- Parthasarathy S, Wendel C, Haynes PL, et al. A pilot study of CPAP adherence promotion by peer buddies with sleep apnea. J Clin Sleep Med 2013;9:543-50. [PubMed]
- Lewis KE, Seale L, Bartle IE, et al. Early predictors of CPAP use for the treatment of obstructive sleep apnea. Sleep 2004;27:134-8. [PubMed]
- Ye L, Pack AI, Maislin G, et al. Predictors of continuous positive airway pressure use during the first week of treatment. J Sleep Res 2012;21:419-26. [PubMed]
- Weaver TE, Maislin G, Dinges DF, et al. Self-efficacy in sleep apnea: instrument development and patient perceptions of obstructive sleep apnea risk, treatment benefit, and volition to use continuous positive airway pressure. Sleep 2003;26:727-32. [PubMed]
- Hoy CJ, Vennelle M, Kingshott RN, et al. Can intensive support improve continuous positive airway pressure use in patients with the sleep apnea/hypopnea syndrome? Am J Respir Crit Care Med 1999;159:1096-100. [PubMed]
- Baron KG, Smith TW, Berg CA, et al. Spousal involvement in CPAP adherence among patients with obstructive sleep apnea. Sleep Breath 2011;15:525-34. [PubMed]
- Glazer Baron K, Gunn HE, Czajkowski LA, et al. Spousal involvement in CPAP: does pressure help? J Clin Sleep Med 2012;8:147-53. [PubMed]
- Toma T, Athanasiou T, Harling L, et al. Online social networking services in the management of patients with diabetes mellitus: systematic review and meta-analysis of randomised controlled trials. Diabetes Res Clin Pract 2014;106:200-11. [PubMed]
- Park C, Kim G, Patel I, et al. Improving adherence to acne treatment: the emerging role of application software. Clin Cosmet Investig Dermatol 2014;7:65-72. [PubMed]
- Tran N, Coffman JM, Sumino K, et al. Patient reminder systems and asthma medication adherence: a systematic review. J Asthma 2014;51:536-43. [PubMed]
- Schwickert L, Becker C, Lindemann U, et al. Fall detection with body-worn sensors: a systematic review. Z Gerontol Geriatr 2013;46:706-19. [PubMed]
- Fox N, Hirsch-Allen AJ, Goodfellow E, et al. The impact of a telemedicine monitoring system on positive airway pressure adherence in patients with obstructive sleep apnea: a randomized controlled trial. Sleep 2012;35:477-81. [PubMed]
- Stepnowsky C, Edwards C, Zamora T, et al. Patient perspective on use of an interactive website for sleep apnea. Int J Telemed Appl 2013;2013:239382.
- Sparrow D, Aloia M, Demolles DA, et al. A telemedicine intervention to improve adherence to continuous positive airway pressure: a randomised controlled trial. Thorax 2010;65:1061-6. [PubMed]
- Anandam A, Akinnusi M, Kufel T, et al. Effects of dietary weight loss on obstructive sleep apnea: a meta-analysis. Sleep Breath 2013;17:227-34. [PubMed]
- Greenburg DL, Lettieri CJ, Eliasson AH. Effects of surgical weight loss on measures of obstructive sleep apnea: a meta-analysis. Am J Med 2009;122:535-42. [PubMed]
- Araghi MH, Chen YF, Jagielski A, et al. Effectiveness of lifestyle interventions on obstructive sleep apnea (OSA): systematic review and meta-analysis. Sleep 2013;36:1553-62, 1562A-1562E.
- Smith PL, Gold AR, Meyers DA, et al. Weight loss in mildly to moderately obese patients with obstructive sleep apnea. Ann Intern Med 1985;103:850-5. [PubMed]
- Iftikhar IH, Kline CE, Youngstedt SD. Effects of exercise training on sleep apnea: a meta-analysis. Lung 2014;192:175-84. [PubMed]
- Dixon JB, Schachter LM, O'Brien PE, et al. Surgical vs conventional therapy for weight loss treatment of obstructive sleep apnea: a randomized controlled trial. JAMA 2012;308:1142-9. [PubMed]
- McEvoy RD, Sharp DJ, Thornton AT. The effects of posture on obstructive sleep apnea. Am Rev Respir Dis 1986;133:662-6. [PubMed]
- Phillips BA, Okeson J, Paesani D, et al. Effect of sleep position on sleep apnea and parafunctional activity. Chest 1986;90:424-9. [PubMed]
- Wenzel S, Smith E, Leiacker R, et al. Efficacy and longterm compliance of the vest preventing the supine position in patients with obstructive sleep apnea. Laryngorhinootologie 2007;86:579-83. [PubMed]
- Sériès F, Marc I. Importance of sleep stage- and body position-dependence of sleep apnoea in determining benefits to auto-CPAP therapy. Eur Respir J 2001;18:170-5. [PubMed]
- Kushida CA, Morgenthaler TI, Littner MR, et al. Practice parameters for the treatment of snoring and Obstructive Sleep Apnea with oral appliances: an update for 2005. Sleep 2006;29:240-3. [PubMed]
- Iftikhar IH, Hays ER, Iverson MA, et al. Effect of oral appliances on blood pressure in obstructive sleep apnea: a systematic review and meta-analysis. J Clin Sleep Med 2013;9:165-74. [PubMed]
- Lam B, Sam K, Mok WY, et al. Randomised study of three non-surgical treatments in mild to moderate obstructive sleep apnoea. Thorax 2007;62:354-9. [PubMed]
- Ferguson KA, Cartwright R, Rogers R, et al. Oral appliances for snoring and obstructive sleep apnea: a review. Sleep 2006;29:244-62. [PubMed]
- Engleman HM, McDonald JP, Graham D, et al. Randomized crossover trial of two treatments for sleep apnea/hypopnea syndrome: continuous positive airway pressure and mandibular repositioning splint. Am J Respir Crit Care Med 2002;166:855-9. [PubMed]
- Ferguson KA, Ono T, Lowe AA, et al. A randomized crossover study of an oral appliance vs nasal-continuous positive airway pressure in the treatment of mild-moderate obstructive sleep apnea. Chest 1996;109:1269-75. [PubMed]
- Barnes M, McEvoy RD, Banks S, et al. Efficacy of positive airway pressure and oral appliance in mild to moderate obstructive sleep apnea. Am J Respir Crit Care Med 2004;170:656-64. [PubMed]
- El-Solh AA, Moitheennazima B, Akinnusi ME, et al. Combined oral appliance and positive airway pressure therapy for obstructive sleep apnea: a pilot study. Sleep Breath 2011;15:203-8. [PubMed]
- Khan A, Ramar K, Maddirala S, et al. Uvulopalatopharyngoplasty in the management of obstructive sleep apnea: the mayo clinic experience. Mayo Clin Proc 2009;84:795-800. [PubMed]
- Mortimore IL, Bradley PA, Murray JA, et al. Uvulopalatopharyngoplasty may compromise nasal CPAP therapy in sleep apnea syndrome. Am J Respir Crit Care Med 1996;154:1759-62. [PubMed]
- Caples SM, Rowley JA, Prinsell JR, et al. Surgical modifications of the upper airway for obstructive sleep apnea in adults: a systematic review and meta-analysis. Sleep 2010;33:1396-407. [PubMed]
- Aurora RN, Casey KR, Kristo D, et al. Practice parameters for the surgical modifications of the upper airway for obstructive sleep apnea in adults. Sleep 2010;33:1408-13. [PubMed]
- Camacho M, Certal V, Brietzke SE, et al. Tracheostomy as treatment for adult obstructive sleep apnea: a systematic review and meta-analysis. Laryngoscope 2014;124:803-11. [PubMed]
- Colrain IM, Black J, Siegel LC, et al. A multicenter evaluation of oral pressure therapy for the treatment of obstructive sleep apnea. Sleep Med 2013;14:830-7. [PubMed]
- Berry RB, Kryger MH, Massie CA. A novel nasal expiratory positive airway pressure (EPAP) device for the treatment of obstructive sleep apnea: a randomized controlled trial. Sleep 2011;34:479-85. [PubMed]
- Rossi VA, Winter B, Rahman NM, et al. The effects of Provent on moderate to severe obstructive sleep apnoea during continuous positive airway pressure therapy withdrawal: a randomised controlled trial. Thorax 2013;68:854-9. [PubMed]
- Jordan AS, White DP, Lo YL, et al. Airway dilator muscle activity and lung volume during stable breathing in obstructive sleep apnea. Sleep 2009;32:361-8. [PubMed]
- Eastwood PR, Barnes M, Walsh JH, et al. Treating obstructive sleep apnea with hypoglossal nerve stimulation. Sleep 2011;34:1479-86. [PubMed]
- Van de Heyning PH, Badr MS, Baskin JZ, et al. Implanted upper airway stimulation device for obstructive sleep apnea. Laryngoscope 2012;122:1626-33. [PubMed]
- Malhotra A. Hypoglossal-nerve stimulation for obstructive sleep apnea. N Engl J Med 2014;370:170-1. [PubMed]
- Strollo PJ Jr, Soose RJ, Maurer JT, et al. Upper-airway stimulation for obstructive sleep apnea. N Engl J Med 2014;370:139-49. [PubMed]
- Weaver TE, Maislin G, Dinges DF, et al. Relationship between hours of CPAP use and achieving normal levels of sleepiness and daily functioning. Sleep 2007;30:711-9. [PubMed]
- Dinges DF, Weaver TE. Effects of modafinil on sustained attention performance and quality of life in OSA patients with residual sleepiness while being treated with nCPAP. Sleep Med 2003;4:393-402. [PubMed]
- Schwartz JR, Hirshkowitz M, Erman MK, et al. Modafinil as adjunct therapy for daytime sleepiness in obstructive sleep apnea: a 12-week, open-label study. Chest 2003;124:2192-9. [PubMed]
- Hirshkowitz M, Black J. Effect of adjunctive modafinil on wakefulness and quality of life in patients with excessive sleepiness-associated obstructive sleep apnoea/hypopnoea syndrome: a 12-month, open-label extension study. CNS Drugs 2007;21:407-16. [PubMed]
- Pack AI, Black JE, Schwartz JR, et al. Modafinil as adjunct therapy for daytime sleepiness in obstructive sleep apnea. Am J Respir Crit Care Med 2001;164:1675-81. [PubMed]
- Williams SC, Marshall NS, Kennerson M, et al. Modafinil effects during acute continuous positive airway pressure withdrawal: a randomized crossover double-blind placebo-controlled trial. Am J Respir Crit Care Med 2010;181:825-31. [PubMed]
- Roth T, White D, Schmidt-Nowara W, et al. Effects of armodafinil in the treatment of residual excessive sleepiness associated with obstructive sleep apnea/hypopnea syndrome: a 12-week, multicenter, double-blind, randomized, placebo-controlled study in nCPAP-adherent adults. Clin Ther 2006;28:689-706. [PubMed]
- Krystal AD, Harsh JR, Yang R, et al. A double-blind, placebo-controlled study of armodafinil for excessive sleepiness in patients with treated obstructive sleep apnea and comorbid depression. J Clin Psychiatry 2010;71:32-40. [PubMed]
- Sullivan CE, Issa FG. Obstructive sleep apnea. Clin Chest Med 1985;6:633-50. [PubMed]
- Young T, Palta M, Dempsey J, et al. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med 1993;328:1230-5. [PubMed]
- Jordan AS, Wellman A, Heinzer RC, et al. Mechanisms used to restore ventilation after partial upper airway collapse during sleep in humans. Thorax 2007;62:861-7. [PubMed]
- Wellman A, Eckert DJ, Jordan AS, et al. A method for measuring and modeling the physiological traits causing obstructive sleep apnea. J Appl Physiol (1985) 2011;110:1627-37. [PubMed]
- Loewen AH, Ostrowski M, Laprairie J, et al. Response of genioglossus muscle to increasing chemical drive in sleeping obstructive apnea patients. Sleep 2011;34:1061-73. [PubMed]
- Younes M. Role of arousals in the pathogenesis of obstructive sleep apnea. Am J Respir Crit Care Med 2004;169:623-33. [PubMed]
- Younes M. Role of respiratory control mechanisms in the pathogenesis of obstructive sleep disorders. J Appl Physiol (1985) 2008;105:1389-405. [PubMed]
- Younes M, Ostrowski M, Thompson W, et al. Chemical control stability in patients with obstructive sleep apnea. Am J Respir Crit Care Med 2001;163:1181-90. [PubMed]
- Malhotra A, Pillar G, Fogel RB, et al. Genioglossal but not palatal muscle activity relates closely to pharyngeal pressure. Am J Respir Crit Care Med 2000;162:1058-62. [PubMed]
- Jordan AS, McSharry DG, Malhotra A. Adult obstructive sleep apnoea. Lancet 2014;383:736-47. [PubMed]
- Kobayashi I, Perry A, Rhymer J, et al. Inspiratory coactivation of the genioglossus enlarges retroglossal space in laryngectomized humans. J Appl Physiol (1985) 1996;80:1595-604. [PubMed]
- Malhotra A, Pillar G, Fogel RB, et al. Pharyngeal pressure and flow effects on genioglossus activation in normal subjects. Am J Respir Crit Care Med 2002;165:71-7. [PubMed]
- McSharry D, O'Connor C, McNicholas T, et al. Genioglossus fatigue in obstructive sleep apnea. Respir Physiol Neurobiol 2012;183:59-66. [PubMed]
- Remmers JE, deGroot WJ, Sauerland EK, et al. Pathogenesis of upper airway occlusion during sleep. J Appl Physiol Respir Environ Exerc Physiol 1978;44:931-8. [PubMed]
- Lo YL, Jordan AS, Malhotra A, et al. Genioglossal muscle response to CO2 stimulation during NREM sleep. Sleep 2006;29:470-7. [PubMed]
- Pillar G, Malhotra A, Fogel RB, et al. Upper airway muscle responsiveness to rising PCO(2) during NREM sleep. J Appl Physiol (1985) 2000;89:1275-82. [PubMed]
- Stanchina ML, Malhotra A, Fogel RB, et al. Genioglossus muscle responsiveness to chemical and mechanical stimuli during non-rapid eye movement sleep. Am J Respir Crit Care Med 2002;165:945-9. [PubMed]
- Mezzanotte WS, Tangel DJ, White DP. Waking genioglossal electromyogram in sleep apnea patients versus normal controls (a neuromuscular compensatory mechanism). J Clin Invest 1992;89:1571-9. [PubMed]
- Bonora M, St John WM, Bledsoe TA. Differential elevation by protriptyline and depression by diazepam of upper airway respiratory motor activity. Am Rev Respir Dis 1985;131:41-5. [PubMed]
- Eckert DJ, White DP, Jordan AS, et al. Defining phenotypic causes of obstructive sleep apnea. Identification of novel therapeutic targets. Am J Respir Crit Care Med 2013;188:996-1004. [PubMed]
- Smith PL, Haponik EF, Allen RP, et al. The effects of protriptyline in sleep-disordered breathing. Am Rev Respir Dis 1983;127:8-13. [PubMed]
- Ren J, Poon BY, Tang Y, et al. Ampakines alleviate respiratory depression in rats. Am J Respir Crit Care Med 2006;174:1384-91. [PubMed]
- Moraes W, Poyares D, Sukys-Claudino L, et al. Donepezil improves obstructive sleep apnea in Alzheimer disease: a double-blind, placebo-controlled study. Chest 2008;133:677-83. [PubMed]
- Eisele DW, Schwartz AR, Smith PL. Tongue neuromuscular and direct hypoglossal nerve stimulation for obstructive sleep apnea. Otolaryngol Clin North Am 2003;36:501-10. [PubMed]
- Oliven A, O'Hearn DJ, Boudewyns A, et al. Upper airway response to electrical stimulation of the genioglossus in obstructive sleep apnea. J Appl Physiol (1985) 2003;95:2023-9. [PubMed]
- Oliven A, Schnall RP, Pillar G, et al. Sublingual electrical stimulation of the tongue during wakefulness and sleep. Respir Physiol 2001;127:217-26. [PubMed]
- Schwartz AR, Bennett ML, Smith PL, et al. Therapeutic electrical stimulation of the hypoglossal nerve in obstructive sleep apnea. Arch Otolaryngol Head Neck Surg 2001;127:1216-23. [PubMed]
- Gleeson K, Zwillich CW, White DP. The influence of increasing ventilatory effort on arousal from sleep. Am Rev Respir Dis 1990;142:295-300. [PubMed]
- Heinzer RC, White DP, Jordan AS, et al. Trazodone increases arousal threshold in obstructive sleep apnoea. Eur Respir J 2008;31:1308-12. [PubMed]
- Eckert DJ, Younes MK. Arousal from sleep: implications for obstructive sleep apnea pathogenesis and treatment. J Appl Physiol (1985) 2014;116:302-13. [PubMed]
- Younes M, Ostrowski M, Atkar R, et al. Mechanisms of breathing instability in patients with obstructive sleep apnea. J Appl Physiol (1985) 2007;103:1929-41. [PubMed]
- Horner RL, Rivera MP, Kozar LF, et al. The ventilatory response to arousal from sleep is not fully explained by differences in CO(2) levels between sleep and wakefulness. J Physiol 2001;534:881-90. [PubMed]
- Trinder J, Ivens C, Kleiman J, et al. The cardiorespiratory activation response at an arousal from sleep is independent of the level of CO(2). J Sleep Res 2006;15:174-82. [PubMed]
- White DP. Pathogenesis of obstructive and central sleep apnea. Am J Respir Crit Care Med 2005;172:1363-70. [PubMed]
- Eckert DJ, Malhotra A, Wellman A, et al. Trazodone increases the respiratory arousal threshold in patients with obstructive sleep apnea and a low arousal threshold. Sleep 2014;37:811-9. [PubMed]
- Veasey SC, Fenik P, Panckeri K, et al. The effects of trazodone with L-tryptophan on sleep-disordered breathing in the English bulldog. Am J Respir Crit Care Med 1999;160:1659-67. [PubMed]
- Berry RB, McCasland CR, Light RW. The effect of triazolam on the arousal response to airway occlusion during sleep in normal subjects. Am Rev Respir Dis 1992;146:1256-60. [PubMed]
- Eckert DJ, Owens RL, Kehlmann GB, et al. Eszopiclone increases the respiratory arousal threshold and lowers the apnoea/hypopnoea index in obstructive sleep apnoea patients with a low arousal threshold. Clin Sci (Lond) 2011;120:505-14. [PubMed]
- Cherniack NS. Respiratory dysrhythmias during sleep. N Engl J Med 1981;305:325-30. [PubMed]
- Gederi E, Nemati S, Edwards BA, et al. Model-based estimation of loop gain using spontaneous breathing: a validation study. Respir Physiol Neurobiol 2014;201:84-92. [PubMed]
- Wellman A, Jordan AS, Malhotra A, et al. Ventilatory control and airway anatomy in obstructive sleep apnea. Am J Respir Crit Care Med 2004;170:1225-32. [PubMed]
- Edwards BA, Sands SA, Owens RL, et al. Effects of hyperoxia and hypoxia on the physiological traits responsible for obstructive sleep apnoea. J Physiol 2014;592:4523-35. [PubMed]
- Wellman A, Malhotra A, Jordan AS, et al. Effect of oxygen in obstructive sleep apnea: role of loop gain. Respir Physiol Neurobiol 2008;162:144-51. [PubMed]
- Edwards BA, Sands SA, Eckert DJ, et al. Acetazolamide improves loop gain but not the other physiological traits causing obstructive sleep apnoea. J Physiol 2012;590:1199-211. [PubMed]