
In-hospital outcome of primary PCI for patients with acute myocardial infarction and prior coronary artery bypass grafting
Introduction
Present guidelines recommend the timely reperfusion therapy for patients with acute myocardial infarction (AMI) to prevent or minimize ischemic complications with antithrombotic medications and/or catheter-based interventions (1,2). The anatomy and blood supply to the coronary artery in patients who had prior coronary artery bypass grafting (CABG) became more complex, when compared to patients without bypass history. It is more difficult to accurately judge culprit vessels in the setting of an emergency angiography, while bypass grafts often play a role. The culprit vessel was identified according to the comprehensive judgement by electrocardiographic findings, current coronary angiograms, and previous bypass angiograms if available. The present study aimed to analyze the primary PCI success rate and in-hospital outcome of patients with prior CABG. The hypothesis was that patients who receive primary PCI with AMI and prior CABG have poor clinical outcomes.
We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/jtd-20-1813).
Methods
Subjects
This was a retrospective study. From January 2011 to December 2018, 3,685 patients received primary coronary angiography in the setting of AMI at Heart Center Beijing of Chaoyang Hospital, including ST-elevation myocardial infarction (STEMI, n=2,587) and non-ST-elevation myocardial infarction (NSTEMI, n=1,098), The data of 78 cases with prior CABG (study group) in 3,685 were retrospectively analyzed. The same number of patients well-matched in spectrum of AMI and three-vessel diseases were selected by random number generator from the same pool as control (control group).
All patients in the study group received successful CABG at least 1 month before the target AMI. The emergency angiography was performed within 12 hours from the onset of STEMI and within 72 hours of NSTEMI. The diagnostic criteria followed the 2017 ESC Guidelines for the management of AMI in patients presenting with ST-segment elevation and the 2015 ESC Guidelines for the management of acute coronary syndromes in patients without persistent ST-segment elevation (1,2). STEMI was defined as acute myocardial ischemia with cardiac troponin I (cTnI) levels >0.5 ng/mL (10-fold of the upper limit of normal) and significant ST-segment elevation (>0.2 mV in two adjacent chest leads and/or >0.1 mV in two adjacent limb leads), or a new left bundle branch block in the electrocardiogram (ECG). NSTEMI was defined as acute myocardial ischemia with cTnI >0.5 ng/mL in the absence of significant ST-segment elevation. Patients were excluded from the analysis when myocardial infarction (MI) occurred during the perioperative period after the CABG, or the clinical record data was not available completely.
Inclusion and exclusion criteria
Inclusion criteria: (I) patients were diagnosed with AMI as definition at admission; (II) age >18 years old; (III) had received emergency coronary angiography within 12 hours from the onset of STEMI and 72 hours of NSTEMI. Extra Inclusion criteria in study group: (I) patients who had received CABG at least 1 month before the target AMI; (II) with negative CABG relevant serum cardiac biomarkers.
Exclusion criteria: (I) patients were excluded from the analysis when the MI occurred during the perioperative period after the CABG; (II) with incomplete clinical record data to analyze.
Definition
Primary PCI success was defined as a restoration of thrombolysis in myocardial infarction (TIMI) 3 blood flow of the culprit vessels combined with symptom relief and the resolution in ST segment in the ECG through coronary angiography procedure and survived to discharge.
The end points
The primary end point was PCI success rate in both groups. The secondary end point was all-cause in-hospital mortality. The PCI success rate and all-cause deaths were calculated during the hospitalization. This index was compared with the control group. The follow-up of hospital events was based on the medical record.
In the present study, the main observational indicators included the medical history of each patient’s hospitalization and their previous CABG surgery records. These records were combined with enhanced coronary arterial computed tomography (CT) imaging to identify the number, site and patency of bypass bridges. Furthermore, the present study analyzed the procedures of emergent angiography and PCI, the symptom relief after intervention, and the changes in ST segment in the ECG, left ventricular ejection fraction (LVEF) by echocardiography, and the peak values of serum cTnI and B-type brain peptide (BNP) during the hospitalization. The clinical follow-up was conducted by telephone or outpatient service.
Ethics approval
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by The Ethics Committee of the Beijing Chaoyang Hospital approved this study (the study approval number (NO.: 2019-scientific-5-1) and individual consent for this retrospective analysis was waived.
Angiographic assessment
The emergency catheterization procedure was performed according to standard techniques. All patients received 80–100 IU of unfractionated heparin before the primary PCI. Basal angiography was obtained in different angiographic views to observe the native coronary arteries and bypass grafts. If necessary, pig-tail catheters were used to detect the bypass grafts.
Statistical analysis
The statistical analysis was performed using SPSS 22.0 (IBM SPSS Statistics; IBM Corporation, Armonk, New York, USA). Normally distributed continuous variables were expressed as mean ± standard deviation, non-normally distributed continuous variables were expressed in median [interquartile range (IQR)], and the categorical variables were expressed in frequency [percentage (%)]. Kolmogorove-Smirnov tests were performed to test the parametric distribution. Either t-test for unpaired samples (parametric distribution), or Manne-Whitney U-test (non-parametric distribution) were performed to test the statistical differences between the two groups, and compare the continuous variables. For categorical variables, chi-square or Fisher’s exact test were carried out to test for significant differences. Multivariate conditional logistic regression models were constructed to test the association between PCI success rate and the prior CABG. All variables were compared using a bilateral test, with P<0.05 considered statistically significant.
Results
General characteristics
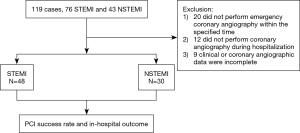
A total of 119 cases with prior CABG were screened. Seventy-eight of 119 cases who achieved the criteria were included, while 41 cases were excluded. Among 41 cases, 20 did not receive emergency coronary angiography within the defined time, 12 did not perform coronary angiography during the hospitalization, and 9 had incomplete clinical or coronary angiographic data for analyzing (Figure 1). Among the included 78 patients, 48 patients had STEMI and 30 patients had NSTEMI. The span from CABG to the index AMI ranged within 1–241 months, with an average of 112.9 months.
In the control group, 78 cases presented with AMI and three-vessel disease, 48 with STEMI and 30 with NSTEMI, without a history of CABG were treated by primary coronary angiographic procedure during the same period as study group.
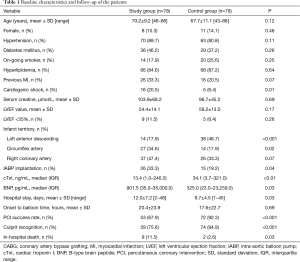
The study group had more incidence of cardiogenic shock, intra-aortic balloon pump (IABP) implantation as well as in-hospital death than the control group, and longer hospital stay. The characteristics and clinical variables are presented in Table 1.
Full table
Angiographic results and interventional procedure
All cases in the study group were performed primary angiography through femoral arterial access: 43 patients from the right and 9 from the left femoral access, 26 from the bilateral femoral access with IABP implantation.
A total of 229 bypass grafts were established in 78 cases in the study group, with an average of 2.93 per case. Among these, 76 bypass grafts were established to the left anterior descending (LAD), in which 74 were left internal mammary artery grafts (LIMA) and 2 were saphenous vein grafts (SVGs), 77 bypass grafts were established to the left circumflex (LCX), in which 4 were radial artery grafts and 73 were SVGs, and 76 bypass grafts were established to the right coronary artery (RCA), and all were SVGs. For 17 (21.8%) patients, the medical team was not able to identify the culprit vessels. Both the native coronary arteries and bypass grafts supply flowed to the electrocardiographic ischemic area, and three patients harvested chest pain relief and electrocardiographic ST-segment resolution after intervention of the presumable culprit vessels.
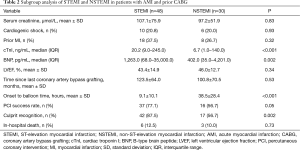
In 30 patients, a drug-eluting stent (DES) was implanted in the culprit lesions.11 patients were treated with drug-coated balloon (DCB) after percutaneous transluminal coronary angioplasty (PTCA) was performed with. In 15 patients, the culprit vessels restored the TIMI 3 flow through PTCA. Thrombus aspiration were performed in 11 patients. Four of 11 suctions of the native coronary artery were performed, and another seven suctions of the bypass grafts were performed, which included one suction using an AngioJet catheter. One patient survived the gastrointestinal bleeding after a transfusion treatment. The subgroup analysis revealed that the STEMI group had a shorter onset to balloon time and more recognition of the culprit, when compared to the NSTEMI group. However, these shared the same in-hospital mortality with each other. It was considered that the STEMI group had a larger MI territory and the worse cardiac function, as indicated by the higher serum concentration of cTnI and BNP (Table 2).
Full table
In the control group, 75 cases were performed primary angiography through right radial arterial access, 3 from the right femoral access.
The culprit lesion in patients with prior CABG
In the study group, 61 cases had explicit culprit vessels: 33 in a native coronary artery and 28 in a bypass graft (42.3% vs. 35.9%, P=0.41). Seventeen cases presented with NSTEMI had ambiguous culprit vessels (21.8%). The culprit lesion in a bypass graft were more frequently spotted in a venous graft than in an arterial graft (89.3% vs. 10.7%, P<0.0001). The primary PCI failed in four cases that presented with STEMI: one case had a culprit lesion in the LCX with severe calcification, and repeated balloon dilatation failed to restore TIMI 3 antegrade blood flow, and stent implantation failed; two cases had culprit lesions in the SVG to RCA, repeated balloon dilatation failed to restore TIMI 3 antegrade blood flow, and stent implantation failed; and in one case the guidewire did not pass through a diffused culprit lesion in the SVG to LCX. A total of 11 PCIs failed (eight cases without angiographic restoration of TIMI 3 flow and three hospital deaths).
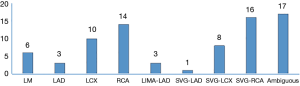
In the study group, more culprit vessels were the SVG-RCA grafts and RCA (band together 38.5%), producing more inferior wall attacks than other territory (Figure 2). In the 48 patients who presented with STEMI, the inferior wall infarction ratio was significantly higher than the anterior wall infarction ratio (56.3% vs. 16.7%, P<0.001). The pie chart presents a description of the distribution of the infarct site (Figure 3).
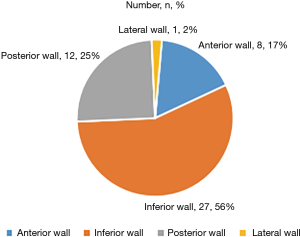
In the control group, the culprit vessels were identified in all cases. LAD territory attack was seen more often than other territory (Table 1).
In-hospital mortality between two groups
The study group had a lower success rate of primary PCI (67.9% vs. 92.3%, P<0.001) and a higher in-hospital mortality than the control group (11.5% vs. 2.5%, P=0.03). Fifty-three in the study group and 72 in the non-CABG succeeded in primary PCI procedure according to the definition. Nine cases died in the study group, 6 STEMI and 3 NSTEMI. Meanwhile, two cases died in the control group, 1 STEMI and 1 NSTEMI (Table 1).
The relationship between PCI success rate and the prior CABG
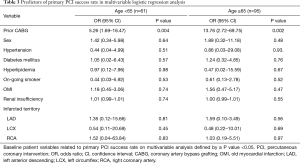
Multivariable modeling revealed the history of prior CABG was the only independent risk factor for emergency PCI success rate other than sex, hypertension, diabetes mellitus, diabetes mellitus, hyperlipidemia, on-going smoker, old myocardial infarction (OMI), renal insufficiency, infarcted territory both in young patients [age <65 years; odds ratio (OR) =5.26, 95% confidence interval (CI): 1.69–16.47] and elderly (age ≥65 years; OR =13.76, 95% CI: 2.72–69.75) (Table 3).
Full table
Discussion
By retrospectively analyzing the primary PCI procedures in 78 patients with AMI and prior CABG, the present observational study revealed the decreased success rate of PCI and the increase in in-hospital mortality in these specific patients with prior CABG, when compared to their counterparts that had no bypass experience. The process of emergency PCI of such patients is more complex and the outcome of hospitalization is poor. The primary PCI success rate is 67.9%, which was significantly lower than the index of the contemporaneous data of patients without prior CABG, according to the hospital’s database (67.9% vs. 92.3%, P<0.001). Meanwhile, the in-hospital mortality was 4.6 times higher than the study counterparts for the same period. These study results are in accordance with the observation of Blachutzi. Both studies exhibited a significant increase in mortality in patients who previously received CABG in the primary PCI of AMI (3). Blachutzi’s observational study included 121 patients with a 13% in-hospital mortality, and this study revealed that poor outcome was mainly associated with older age and congestive heart failure (3). The CAMI registered study that looked at years 2013–2014 revealed that 8% Chinese patients hospitalized for AMI (including STEMI and NSTEMI) had a prior MI (4). A research for reperfusion treatment for AMI from a central city in China revealed that 14.7% of the enrolled STEMI patients had a prior MI (5). However, in the present study, 33.3% patients had a prior MI before they received CABG. This means that more objects in the study were in a status of chronic heart failure. Recurrent MI, even with a smaller territory, is prone to induce heart failure or cardiogenic shock (6). In the present study, the BNP peak values, but not the cTnI, were significantly higher in the death group, when compared to the surviving group. The high BNP elevation reflected the degree of insufficiency of heart function, which is positively correlated to the mortality during hospitalization (7). Although the serum cTnI were lower in the study group, the clinical outcomes were worse. This suggests a history of prior CABG is a predictor for a worse in-hospital outcome for patients who underwent primary PCI even with less infarcted territory. Two clinical researches conducted by Le-feng Wang and Li Xu revealed that cardiogenic shock is an independent predictor of death during hospitalization for AMI treated with emergency PCI (8,9). Consistent with these conclusions, the present study also revealed that cardiogenic shock increased the risk of death of high-risk AMI patients treated by emergency PCI by 4.6 times. The subgroup analysis for the patients with prior CABG revealed that the STEMI group had a shorter onset to balloon time and more recognition of culprit, when compared to the NSTEMI group, but these two groups shared the same in-hospital mortality. It was considered that the STEMI group has a larger MI territory and the worse cardiac function, as indicated by the higher serum concentration of cTnI and BNP. The present observational study also revealed that patients who had prior CABG and developed STEMI had a more significant inferior wall, when compared to the anterior wall. Through case analysis, it was observed that 97.4% of the bridges to the LAD were arterial grafts, and this was significantly higher, when compared to arterialization grafts for LCX (5.2%, P<0.0001) and RCA (0%, P<0.0001). During the average of 112.9 months of time since the last CABG to enrollment, the patent rate was 83.8% for the LIMA bridge, 50% for the SVG-LCX bridge, and 31.4% for the SVG-RCA bridge. Yi et al. reported that the long-term patent rate for an arterial graft is significantly higher than the venous one (10). This asserts that the less anterior wall attacks are linked to the low closure rate of LIMA bridges. In the present study, it was found that more culprit lesions in the SVG-RCA grafts and RCA (band together at 38.5%) caused more inferior wall attacks. Blachutzi’s study revealed that the culprit lesions in patients with prior CABG that presented with AMI were more often located in bypass grafts than in native arteries (3). However, the present study revealed that there was no significant difference between bypass grafts and the native coronary arteries in their contribution to be a culprit. However, the study conducted by Blachutzi and the present study analyzed a small sample of patients, and it was considered that more researches need to be performed to clarify the truth. The operator in an emergent setting often meets more technical and strategical difficulties in performing a PCI for cases with prior CABG due to the lack of information of the previous bypass and native vessels (11-14). In one case, the patient experienced STEMI in the anterior wall, with the left main trunk and three vessels disease by coronary angiography, and received staged CABG thereafter. A second STEMI occurred in the inferior wall at three months after the bypass surgery. The emergency angiography revealed an occlusion both in the middle site of the RCA and proximal site of the SVG graft. The PCI shifted to the SVG graft after a failed attempt to intervene the RCA. A Y-type SVG graft emerged after balloon dilation and aspiration, supplying flow to the RCA and LCX. The culprit of the bypass graft restored the TIMI 3 blood flow, and the elevated ST segment in the ECG was resolved. This case reflects the complexity of the emergency PCI procedure in patients with prior CABG. The operator prefers to choose a technically toilless candidate culprit to perform when there is more than one potential vessel. Misjudging often delays the time of reperfusion. The present study shows that emergency PCI for patients with prior CABG is a procedure with a high risk of mortality and technical complexity. The present group of patients with previous CABG was older, with more prior MI and renal insufficiency. AMI was more likely to induce acute congestive heart failure (15,16) and aggravate the renal injury (17-19). The possible reasons for the lower level of LVEF in this group of patients were the less viable myocardium in some parts of the cases, and the progression of the ischemic cardiomyopathy (20). Therefore, primary PCI for patients with AMI and prior CABG induces more adverse events during hospitalization, which remains as a challenge for interventional cardiologists.
Limitations
There were several limitations in the present study. First, the present study was non-randomized controlled trial. Second, the present study was merely a single-center trial, and the sample size was limited. Third, the clinical follow-up was short, and it was necessary to observe the clinical long-term prognosis. Conclusion: Patients who underwent a primary PCI with AMI and prior CABG have poor in-hospital outcomes, low PCI success rates, and high mortality.
Acknowledgments
Funding: This original article is sponsored by the Beijing Municipal Administration of Hospitals Digest Collaborative Center major project (XXZ0607).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/jtd-20-1813
Data Sharing Statement: Available at http://dx.doi.org/10.21037/jtd-20-1813
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd-20-1813). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by The Ethics Committee of the Beijing Chaoyang Hospital approved this study (the study approval number (NO.: 2019-scientific-5-1) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ibanez B, James S, Agewall S, et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J 2018;39:119-77. [Crossref] [PubMed]
- Roffi M, Patrono C, Collet JP, et al. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC). Eur Heart J 2016;37:267-315. [Crossref] [PubMed]
- Blachutzik F, Achenbach S, Troebs M, et al. Angiographic Findings and Revascularization Success in Patients With Acute Myocardial Infarction and Previous Coronary Bypass Grafting. Am J Cardiol 2016;118:473-6. [Crossref] [PubMed]
- Xu H, Li W, Yang J, et al. The China Acute Myocardial Infarction (CAMI) Registry: A national long-term registry-research-education integrated platform for exploring acute myocardial infarction in China. Am Heart J 2016;175:193-201.e3. [Crossref] [PubMed]
- Zhao X, Yang X, Gao C, et al. Improved Survival of Patients with ST-Segment Elevation Myocardial Infarction 3-6 Hours After Symptom Onset Is Associated with Inter-Hospital Transfer for Primary Percutaneous Coronary Intervention (PCI) at a Large Regional ST-Segment Elevation Myocardial Infarction (STEMI) Program vs. In-Hospital Thrombolysis in a Community Hospital. Med Sci Monit 2017;23:1055-63. [Crossref] [PubMed]
- Hellermann JP, Goraya TY, Jacobsen SJ, et al. Incidence of heart failure after myocardial infarction: is it changing over time? Am J Epidemiol 2003;157:1101-7. [Crossref] [PubMed]
- McCullough PA, Nowak RM, McCord J, et al. B-type natriuretic peptide and clinical judgment in emergency diagnosis of heart failure: analysis from Breathing Not Properly (BNP) Multinational Study. Circulation 2002;106:416-22. [Crossref] [PubMed]
- Wang L, Zhang D, Yang X, et al. Efficacy of primary PCI for acute myocardial infarction due to abrupt occlusion of left main trunk. Chinese Journal of Cardiology 2012;40:813-6. [PubMed]
- Xu L, Wang L, Yang X, et al. Analysis of acute myocardial infarction complicated with cardiogenic shock due to unprotected left main coronary artery disease. Chinese Journal of Emergency Medicine 2015;24:735-40.
- Yi G, Shine B, Rehman SM, et al. Effect of bilateral internal mammary artery grafts on long-term survival: a meta-analysis approach. Circulation 2014;130:539-45. [Crossref] [PubMed]
- Pillai AA, Saranya GV, Selvaraj RJ, et al. Intra vascular ultrasound findings in drug eluting stent restenosis following emergent PCI for acute coronary syndrome – Gender based analysis. Journal of Indian College of Cardiology 2017;7:78-84. [Crossref]
- . TCTAP C-030 Rescue from Left Main Coronary Artery Bifurcation Spiral Wu HP. Dissection During Emergent PCI Procedure. J Am Coll Cardiol 2015;65:S127-9. [Crossref]
- Mogi S, Kohsaka S, Maekawa Y, et al. In-hospital outcome of emergent PCI after cardiopulmonary arrest due to acute coronary syndrome. European Heart Journal 2013;34:4043. [Crossref]
- Hershey J, Isada L, Fenster MS. Emergent primary PCI of anomalous LAD. J Invasive Cardiol 2006;18:E152-3. [PubMed]
- Kim CS, Kim MJ, Kang YU, et al. Influence of renal dysfunction on clinical outcomes in patients with congestive heart failure complicating acute myocardial infarction. Int Heart J 2013;54:304-10. [Crossref] [PubMed]
- Nallamothu BK, Wang Y, Cram P, et al. Acute myocardial infarction and congestive heart failure outcomes at specialty cardiac hospitals. Circulation 2007;116:2280-7. Erratum in: Circulation 2008;117:e10. [Crossref] [PubMed]
- Yesin M, Kalçık M, Gürsoy MO, et al. Acute myocardial infarction in a patient suffering from penicillin-induced laryngeal edema: Kounis syndrome aggravated by adrenaline. Wien Klin Wochenschr 2017;129:509-11. [Crossref] [PubMed]
- Abusaada K, Yuan C, Sabzwari R, et al. Development of a novel score to predict the risk of acute kidney injury in patient with acute myocardial infarction. J Nephrol 2017;30:419-25. [Crossref] [PubMed]
- Liao Y, Dong X, Chen K, et al. Renal function, acute kidney injury and hospital mortality in patients with acute myocardial infarction. J Int Med Res 2014;42:1168-77. [Crossref] [PubMed]
- Berry C, Pieper KS, White HD, et al. Patients with prior coronary artery bypass grafting have a poor outcome after myocardial infarction: an analysis of the VALsartan in acute myocardial iNfarcTion trial (VALIANT). Eur Heart J 2009;30:1450-6. [Crossref] [PubMed]


