Surgical treatment for pulmonary tuberculosis: is video-assisted thoracic surgery “better” than thoracotomy?
Introduction
Currently, the treatment of pulmonary tuberculosis (TB) is medical in the majority of cases; however, the disease has been treated operatively for more than 70 years and many surgical procedures have been developed for the treatment of pulmonary TB (1-6). Indications for surgery in patients with pulmonary TB have changed markedly with the development of anti-TB drugs and advances in surgical techniques (1-6). Though medical therapy is successful in most cases, approximately 5% of patients with pulmonary TB require surgery (1,4,7).
Video-assisted thoracoscopic surgery (VATS) was first reported in 1992, and since its introduction it has greatly improved surgical outcomes for many thoracic diseases and has become the preferred thoracic surgical approach in developed countries. VATS lobectomy has now become a major surgical treatment for lung cancer patients and is an effective method for treating early lung cancer, with more than 80% of lobectomies being performed with VATS (8-12). The procedure was introduced in China in 2006, and is now widely used for treating thoracic diseases.
Tuberculous lesions in the lung that require surgical treatment are in many cases associated with pleural and vascular adhesions, and treatment has traditionally been with open thoracotomy (1,4). With advances in medical technology and accumulated experience with VATS, studies have reported that VATS can be safe and effective for the management of pulmonary TB that requires surgery (13-16).
The purpose of this study was to compare the results of VATS lobectomy and conventional open lobectomy in patients with pulmonary TB who require surgery.
Patients and methods
Patients
The patients with pulmonary TB were included or not which were decided independently for surgical indications and inclusion and exclusion criteria. Forty patients with pulmonary TB who required lobectomy and were treated at Beijing Chest Hospital between 2008 and 2011 were included in the present study. The patients were randomized divided into two groups: VATS group who received VATS lobectomy and conventional open lobectomy group by random number table method. The study was approved by the local ethics committee, and all enrolled patients provided written informed consent.
Surgical indications
Indication for the surgery in patients with pulmonary TB: (I) tuberculosis empty: TB empty has not been cured or continuous or intermittent positive sputum after initial medical treatment or retreatment specification more than a year. Especially, the patients with drug-resistant Mycobacterium TB should be the preferred surgical treatment; (II) tuberculoma: the small amount of hemoptysis or recurrent hemoptysis, or invalid medication; (III) lung damage; (IV) tuberculous bronchial stenosis or bronchiectasis: repeated emoptysis, repeated infection, sputum positive or pulmonary atelectasis; (V) repeated or prolonged hemoptysis in the patients with pulmonary TB, invalid medication, potentially life-threatening; (VI) persistent, recurrent and limited leaf of the chronic fiber caseous TB.
Inclusion and exclusion criteria
Patients were included in the present study if they met the following criteria: (I) lesions confined to only one lobe; (II) tuberculoma or tuberculous cavity diameter either <4 cm or complicated by bronchiectasis; (III) bronchoscopic examination revealed endobronchial TB without pleural effusion or pleural thickening; (IV) cardiopulmonary function adequate for surgery; (V) preoperative evaluation revealed no severe pleural adhesions or close adhesions between the pulmonary artery, and no calcified or enlarged lymph nodes by chest computed tomography. Exclusion criteria were: (I) history of pleural disease, bronchopleural fistula, whole lung affected by TB, endobronchial TB with pleural effusion or pleural thickening, or prior thoracic surgery; (II) 2 or more lobes affected by TB; (III) preoperative evaluation revealed severe pleural or close adhesions between the pulmonary artery and/or calcified or enlarged lymph nodes by chest computed tomography.
Surgical procedure
Patients were placed in the full lateral decubitus position, general anesthesia was induced, and double-lumen endotracheal intubation with contralateral single lung ventilation was performed. For VATS lobectomy, three incisions were made. The observation port was placed at the junction of the midaxillary line and the seventh or eighth intercostal space. The second incision was made at the lower angle of the scapula in either the seventh, eighth, or ninth intercostal space. A third incision of 3-4 cm in length for the main port was made at the junction of the anterior axillary line and either the fourth, fifth, or sixth intercostal space. The location, extent, and the relationship of the lesion with adjacent tissues were carefully examined after thoracoscope insertion to determine whether the operation could be performed by VATS. The process of dividing the pulmonary vessels is shown in Figure 1A,B. In the majority of patients the pulmonary artery was first ligated, and then the pulmonary vein and lobar bronchus were ligated. In the patients with adhesions between the pulmonary artery and adjacent lymph nodes, the pulmonary vein was ligated first followed by ligation of the lobar bronchus and pulmonary artery. For patients with close adhesions between the pulmonary artery and adjacent lymph nodes, the main port incision was extended to 5-6 cm and a thoracoscope-assisted mini-incision pulmonary lobectomy was performed.
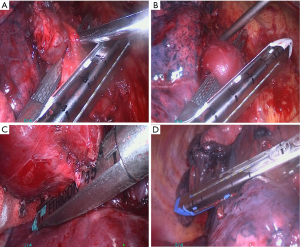
The process of dividing the bronchus is shown in Figure 1C. The lymphatic and fibrous tissues nearest to the bronchus were removed first, and the bronchus was ligated at the bronchial opening to avoid a long bronchial stump. For patients with either pulmonary fissure agenesis or adhesions between lymph nodes and the pulmonary artery, the pulmonary vein was divided first, followed by lobar bronchus and pulmonary artery division (Figure 1D). The specimen was put into an endobag, and then retrieved from the main port. After assuring hemostasis, the wounds were closed in a standard manner.
Conventional open lobectomy was performed in all patients using standard surgical technique.
Outcome measures
Patient demographic data, pulmonary function, type of lung resection, surgery completion rate, intraoperative blood loss, operation time, complications, 24 hours postoperative visual analogue scale (VAS) pain score, duration of pleural cavity drainage, the volume of postoperative chest drainage, postoperative hospital stay and the negative sputum conversion rate were compared between the two groups.
Statistical analysis
The data are presented as frequency and percentage for categoric variables and as median and range for continuous variables. The Wilcoxon rank test were used to compare continuous variables and the χ2 or Fisher exact tests were used for categorical variables and a P value less than 0.05 was considered to be significant. All statistical analysis was performed using the SPSS 13.0 software package (SPSS Inc., Chicago, IL, USA).
Results
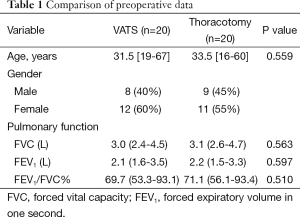
There were 20 patients who received VATS lobectomy and 20 that received conventional open lobectomy, and the two groups were similar with respect to gender, age and pulmonary function (all, P>0.05, Table 1). Operative and postoperative data of the TWO groups is shown in Table 2.
Full table

Full table
In the VATS group there were 8 males and 12 females with a median age of 31.5 years (range, 19-67 years). There were 11 patients with tuberculoma, 2 patients with TB complicated by aspergilloma, 2 patients with TB complicated by bronchiectasis, and 5 patients with tuberculous cavities. Sixteen patients received preoperative anti-TB treatment for more than 6 months, while 4 patients did not receive anti-TB treatment. Eight patients had a right upper lobectomy, 2 patients had a right lower lobectomy, 8 patients had a left upper lobectomy, and 2 patients had a left lower lobectomy.
In the conventional open lobectomy group there were 9 males and 11 females, with a median age of 33.5 years (range, 16-60 years). There were 9 patients with tuberculoma, 4 with TB complicated by aspergilloma, 1 with TB complicated by bronchiectasis, and 6 with tuberculous cavities. Sixteen patients received preoperative anti-TB treatment for more than 6 months, and 4 did not receive anti-TB treatment. Six patients had a right upper lobectomy, 3 patients had a right lower lobectomy, 9 patients had a left upper lobectomy, and 2 patients had a left lower lobectomy.
Lobectomy was completed by VATS in 19 of the 20 patients in the thoracoscopic surgery group (95%), and by thoracoscope-assisted mini-incision lobectomy in 1 patient due to close adhesions between the pulmonary artery and calcified lymph nodes. The median intraoperative blood loss was 345 mL (range, 100-800 mL), and the median duration of pleural cavity closed drainage was 5 days (range, 3-7 days). Postoperative, no TB was identified in patients with positive sputum smears prior to the operation. There were no complications such as postoperative bronchopleural fistula, empyema, or atelectasis, no perioperative deaths, and all patients recovered well.
All open lobectomies were completed successfully, and the median intraoperative blood loss was 445 mL (range, 150-950 mL) and the median duration of pleural cavity closed drainage was 5 days (range, 3-9 days). Postoperatively, no TB was identified in patients with positive sputum smears prior to the operation. No complications such as postoperative bronchopleural fistula, empyema, or atelectasis occurred, there were no perioperative deaths, and all patients recovered well. One patient experienced incisional fatty necrosis which resolved with routine wound care. Two patients developed atelectasis which resolved with bronchoscopic suctioning. Two patients developed arrhythmias which resolved with treatment.
No statistically significant differences were found between the two groups with respect to operation completion rates, pulmonary function, intraoperative blood loss, duration of pleural cavity drainage, the volume of postoperative chest drainage (Tables 1,2), and the negative sputum conversion rate (Table 3). However, the operation time, number of postoperative complications, postoperative pain index at 24 hours after surgery and postoperative hospital stay were all significantly less in the VATS group (Table 2).

Full table
The median follow-up duration was 14 months (range, 8-18 months), and during follow-up there were no positive sputum examination results in either group. No severe complications such as secondary hemothorax, bronchopleural fistula, empyema, respiratory failure, or disseminated TB were noted.
Discussion
The results of this study indicate that VATS lobectomy can be used for treating patients with pulmonary TB who require surgery, and has advantages over open lobectomy including smaller incisions, fewer complications, shorter operation time, less postoperative pain and shorter postoperative hospital stay. Furthermore, postoperative no TB was identified in patients with positive sputum smears prior to the operation in either group.
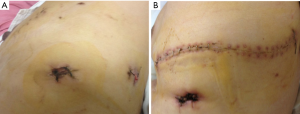
Pulmonary TB is a chronic disease caused by Mycobacterium TB, and the main pathological characteristics include exudation, caseous necrosis, and proliferation, all of which can be found in a single lesion (1). Exudation is very common in pulmonary TB, and pleural adhesions will occur when the pleura is involved in the inflammatory responses (1). These issues make it very difficult to treat pulmonary TB operatively because of the difficulty in dividing the adhered tissues. Conventional open lobectomy can be used to treat pulmonary TB; however, it involves large incisions (Figure 2A) though multiple muscles resulting in significant pain and morbidity. In contrast, VATS lobectomy only requires several small incisions (Figure 2B), it does not involve the latissimus dorsi, costoscapularis, ectopectoralis, or entopectoralis muscles, and the scapulae and ribs remain intact. VATS lobectomy is a relatively simple procedure that requires less time, and is associated with less surgical trauma than conventional thoracotomy.
VATS has become a widely used and effective method for treating thoracic disease (8-11,17). However, lobectomy performed by video-assisted mini-thoracotomy (VAMT) is the most commonly used method for treating pulmonary TB. VATS reduces the invasiveness of surgery still further and can be an effective method in challenging patient groups such as the elderly, debilitated, and those with poorly functioning cardiopulmonary systems. While a small incision is needed for VATS specimen retrieval, the incision is much smaller than that used in conventional thoracotomy and is thus associated with less pain, morbidity and postoperative hospital stay (17).
Studies have indicated that VATS can be effective for patients with pulmonary TB that require surgical treatment. Laisaar et al. (13) reported the results of 43 patients that received thoracoscopic resection of tuberculoma between 1996 and 2008. While three cases required conversion to thoracotomy there were no serious operative or perioperative complications and with a mean follow-up of 9 years none of the patients had a relapse. Hsu et al. (15), in a series of 53 cases, also reported that VATS was useful for the diagnosis and treatment of tuberculoma. Yen et al. (14) retrospectively reviewed the records of patients with pulmonary TB who required a therapeutic lung resection and underwent either VATS or thoracotomy. Of 123 patients, 63 were successfully treated with VATS and 60 required conversion to thoracotomy. VATS was associated with significantly less blood loss, shorter hospital stay, and fewer complications than thoracotomy. The analysis also showed that lesions that required a pneumonectomy or thoracoplasty could not be completed with VATS. In a number of our patients, the TB was complicated by aspergilloma. Yuan et al. (18) reported successful lung resections in 16 patients with both simple and complex aspergilloma.
For VATS, we prefer two incisions of 1.5 cm that are placed based on radiographic data and the patient’s body contour. The first incision is located at the junction of the midaxillary line and the seventh to eighth intercostal space and is used as an observation port. The second incision is located at the lower angle of the scapula in the seventh to ninth intercostal space to help observe diaphragm and costophrenic angle adhesions. A third 3-4 cm incision is located at the junction of the anterior axillary line and the fourth to sixth intercostal space, and is used to conduct the operation. The incisions are spaced apart from each other which prevent the devices from interfering with each other. The main port is located on the anterior axillary line, which assists in dissection of the hilum, pulmonary vessels, and bronchus.
In this study, strict criteria were used for patients with pulmonary TB to be eligible for VATS lobectomy. While severe adhesions are generally not suitable for treatment by VATS, thoracoscopy can provide a wide visual field without an obvious blind area and dividing moderate adhesions is not difficult. For these reasons, VATS can be used in the majority of cases. When adhesions are present, they can generally be divided with an ultrasonic knife. The ultrasonic knife generates much less heat than a high-frequency electrotome, and provides simultaneous division and hemostasis without damaging adjacent tissues. Even blood vessels measuring as small as 3 mm can be solidified by the ultrasonic knife without eschar avulsion bleeding.
In general, we treat the pulmonary artery before the pulmonary vein, which is in agreement with other clinicians (8,9). However, in patients with pulmonary fissure agenesis, pulmonary artery abnormalities, or an excessive number of branch arteries, the lobar bronchus was resected first followed by the pulmonary artery and pulmonary vein. The patients with calcified lymph nodes were excluded because calcified lymph nodes always tell us that some adhesions may appear and surgery by VATS is difficult for patients with calcified lymph nodes. Dense adhesion between calcified lymph nodes and the pulmonary artery may be present if the disease course has been long, and uncontrollable bleeding from the pulmonary artery can occur during dissection. In this case, open thoracotomy or thoracoscopy assisted mini-incision pulmonary lobectomy should be performed. For patients with pulmonary fissure agenesis, an incision of the pulmonary parenchyma should be performed carefully after positioning the pulmonary veins to determine the location of the pulmonary fissure to avoid the pulmonary arteries.
The primary limitation of this study is the small number of patients. However, pulmonary TB is currently primarily treated medically and the number of patients that require surgery is small.
Conclusions
VATS lobectomy is an effective and safe procedure for patients with pulmonary TB that require surgery. It has advantages over open lobectomy including smaller incisions, fewer complications, shorter operation time, less postoperative pain, and postoperative hospital stay and has a similar cure rate as open lobectomy.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Cummings I, O’Grady J, Pai V, et al. Surgery and tuberculosis. Curr Opin Pulm Med 2012;18:241-5. [PubMed]
- Takeda S, Maeda H, Hayakawa M, et al. Current surgical intervention for pulmonary tuberculosis. Ann Thorac Surg 2005;79:959-63. [PubMed]
- Byun CS, Chung KY, Narm KS, et al. Early and Long-term Outcomes of Pneumonectomy for Treating Sequelae of Pulmonary Tuberculosis. Korean J Thorac Cardiovasc Surg 2012;45:110-5. [PubMed]
- Kilani T, Boudaya MS, Zribi H, et al. Surgery for thoracic tuberculosis. Rev Pneumol Clin 2015;71:140-58. [PubMed]
- Kerti CA, Miron I, Cozma GV, et al. The role of surgery in the management of pleuropulmonary tuberculosis - seven years' experience at a single institution. Interact Cardiovasc Thorac Surg 2009;8:334-7; discussion 337. [PubMed]
- Bouchikh M, Achir A, Caidi M, et al. Role of pulmonary resections in management of multidrug-resistant tuberculosis. A monocentric series of 29 patients. Rev Pneumol Clin 2013;69:326-30. [PubMed]
- Botianu PV, Dobrica AC, Butiurca A, et al. Complex space-filling procedures for intrathoracic infections - personal experience with 76 consecutive cases. Eur J Cardiothorac Surg 2010;37:478-81. [PubMed]
- Papiashvilli M, Stav D, Cyjon A, et al. Lobectomy for non-small cell lung cancer: differences in morbidity and mortality between thoracotomy and thoracoscopy. Innovations (Phila) 2012;7:15-22. [PubMed]
- McKenna RJ Jr, Houck W, Fuller CB. Video-assisted thoracic surgery lobectomy: experience with 1,100 cases. Ann Thorac Surg 2006;81:421-5; discussion 425-6. [PubMed]
- Nakanishi K. Video-assisted thoracic surgery lobectomy with bronchoplasty for lung cancer: initial experience and techniques. Ann Thorac Surg 2007;84:191-5. [PubMed]
- Detterbeck F. Thoracoscopic versus open lobectomy debate: the pro argument. Thorac Surg Sci 2009;6:Doc04. [PubMed]
- Begum S, Hansen HJ, Papagiannopoulos K. VATS anatomic lung resections-the European experience. J Thorac Dis 2014;6 Suppl 2:S203-10. [PubMed]
- Laisaar T, Viiklepp P, Hollo V. Long-term follow-up after thoracoscopic resection of solitary pulmonary tuberculoma. Indian J Tuberc 2014;61:51-6. [PubMed]
- Yen YT, Wu MH, Lai WW, et al. The role of video-assisted thoracoscopic surgery in therapeutic lung resection for pulmonary tuberculosis. Ann Thorac Surg 2013;95:257-63. [PubMed]
- Hsu KY, Lee HC, Ou CC, et al. Value of video-assisted thoracoscopic surgery in the diagnosis and treatment of pulmonary tuberculoma: 53 cases analysis and review of literature. J Zhejiang Univ Sci B 2009;10:375-9. [PubMed]
- Beshay M, Dorn P, Kuester JR, et al. Video thoracoscopic surgery used to manage tuberculosis in thoracic surgery. Surg Endosc 2005;19:1341-4. [PubMed]
- Akiba T, Marushima H, Kawahara H, et al. Video-assisted thoracic surgery for patients with lung cancer and interstitial pneumonia. Ann Thorac Cardiovasc Surg 2010;16:236-41. [PubMed]
- Yuan P, Wang Z, Bao F, et al. Is video-assisted thoracic surgery a versatile treatment for both simple and complex pulmonary aspergilloma? J Thorac Dis 2014;6:86-90. [PubMed]


