
The predictive value of Holter monitoring in the risk of obstructive sleep apnea
Introduction
Obstructive sleep apnea (OSA) is a common disease with high morbidity (1) though frequently underdiagnosed (2). The prevalence of OSA is higher in patients with cardiovascular diseases such as hypertension, coronary heart disease, and arrhythmia, and can affect the occurrence and development of cardiovascular diseases (3-5). In addition to snoring, suffocating at night, abrupt awakening, and daytime sleepiness, OSA also often manifests as cardiovascular symptoms (6) such as chest tightness, nocturnal arrhythmia, nocturnal angina pectoris, and refractory hypertension. Patients usually visit a cardiologist, ignoring the symptoms of OSA, which can lead to a missed diagnosis or misdiagnosis.
A Holter monitor is a wearable electrocardiogram (ECG) that is commonly used to assess patients with suspected cardiovascular diseases. Its technology is mature and has been widely promoted and applied in various clinic and hospital settings. In recent years, studies have attempted using ECG-derived respiratory waveforms and heart rate variability (HRV) to diagnose OSA (7,8). However, ECG-derived respiratory waveforms need specific device and software, which was not available in most commercially used Holter monitors; there was no clear cutoff points or algorithms to use HRV indices to diagnose OSA, the development of ECG-based diagnostic tools for OSA is limited in clinical application. In this study, by comparing Holter monitoring to polysomnography (PSG), we try to find out an operable way for clinicians to use Holter to predict OSA risk, thus decrease the miss diagnosis and misdiagnosis of OSA.
We present the following article in accordance with the STARD reporting checklist (available at
Methods
Participants
The participants were recruited consecutively from among patients in a sleep clinic in Guangdong Provincial People’s Hospital who were suspected of OSA from February 2015 to February 2016. Inclusion criteria included: male or female gender and age between 18 and 80 years. Exclusion criteria included: a history of severe hypertension; acute myocardial infarction; unstable angina pectoris; acute stroke; depression, schizophrenia, or other psychotic disorders; severe lung disease requiring oxygen therapy; severe liver or kidney dysfunction; or other conditions that the researchers considered not appropriate for participation in the study. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Informed consent was taken from all the patients. Ethics approval for this study was obtained from the ethics committee of Guangdong Provincial People’s Hospital (No. GDREC2019757H).
Collection of medical history, symptoms, and signs
Symptom evaluation included a history of snoring, difficulty in falling asleep, nocturia, dry mouth in the morning, insomnia, and sexual dysfunction. Medical history obtained from the medical records of the hospital included a history of smoking and alcohol use. The Epworth Sleepiness Scale was used for daytime sleepiness assessment; scores range from 0 to 24, with higher scores indicating increased daytime sleepiness. Physical parameters, such as height, weight, and body mass index (BMI), were also collected.
Holter monitoring and calculation
Holter examination was performed with a new 12-lead 24-hour Holter recorder [DMS300-4AL, DM Systems (Beijing) Co., Ltd., DMS]. The Holter data was interpreted by an experienced ECG specialist, who could not access to the results of the PSG. Holter recordings were used to calculate respiratory waveforms and HRV indices, and DM software was used to derive heart rate trend charts and respiratory waveforms from which sleep apnea or hypopnea could be determined.
Heart rate trend charts were calculated with signal from standard Holter ECG electrodes, and no additional electrodes were needed. Holter breathing events were calculated as follows: three or more consecutive equal-spaced peaks and troughs appeared in the heart rate trend chart, three or more consecutive equal-spaced apnea or hypoventilation morphology appeared in the respiratory waveforms; these two appearing and repeating more than five times was defined as an apnea event. The total time of apnea events (Ta) and the ratio of the apnea events time to the recording time (Ta/Tm) were derived from Holter respiratory waveforms.
Holter HRV indicators included both time and frequency domain measures. The time domain indices included: the standard deviation of all NN (or R-R) intervals during 24 hours (SDNN-24 h); the mean of the standard deviation of NN intervals in all 5-minute segments (SDNN index); standard deviation of the averages of NN intervals in 5-minute segments of the entire recording (SDANN), and the mean of SDANN in all 5-minute segments (SDANN index); the square root of the mean squared differences of successive NN intervals (rMSSD); and the percentage of beat-to-beat NN interval differences that were more than 50 milliseconds (pNN50). Frequency domain indices included: the total spectral power of 24 hours (TF); the minimum spectral power per hour (SPmin); the maximum spectral power per hour (SPmax); very low frequency (VLF), power in the frequency range of 0.003–0.040 Hz; low frequency (LF), power in the frequency range of 0.04–0.15 Hz; and high frequency (HF), power in the frequency range of 0.15–0.4 Hz.
Sleep monitoring and diagnostic standards
Philips Alice5 polysomnography (Philips Respironics, USA) was used for OSA diagnosis. The patient did not take a nap on the day of monitoring and did not drink alcohol, strong tea, coffee, and the like that would interfere with sleep, nor did they take sleeping pills. Monitoring started at 22:00 at the same day of Holter monitoring, and ended at 06:30 the next day. The collection channels included electroencephalogram, electrooculogram, ECG, mandibular electromyogram, thoracoabdominal movement, oral and nasal airflow, finger oxygen saturation, and snoring. Experienced technicians then interpreted the recordings according to the American Academy of Sleep Medicine (AASM) criteria (9). The apnea-hypopnea index (AHI), oxygen reduction index (ODI), mean oxygen saturation at night (SpO2_M), lowest oxygen saturation at night (SpO2_L), time of oxygen saturation less than 90% (T<90%), and the percentage of sleep time when the oxygen saturation below 90% (T<90%/Ts) were recorded. As we know, an AHI ≥5/h is the threshold for diagnosis of OSA, and AHI ≥15/h is the threshold for moderate-to-severe OSA. As moderate-to-severe OSA was more concerned in clinical setting, and more intensive for treatment, here we aim to use Holter to identify and predict moderate-to-severe OSA. Thus, here an AHI ≥15 was defined as OSA, and an AHI <15 was non-OSA (10). PSG technicians were blinded to the results of Holter monitoring.
Statistical analysis
Analyses were conducted using SPSS 25.0 software (IBM SPSS Statistics for Windows; Armonk, NY, USA). Quantitative data that conformed to the normal distribution are presented as mean ± standard deviation. For normally distributed data, t-tests were used to compare the average of two independent samples, and a Pearson correlation analysis was used to determine the correlation between the two sets of data. Quantitative data that are not normally distributed are presented as median and 25% and 75% quartiles. For non-normally distributed data, the equivalent non-parametric analyses were conducted, including Wilcoxon Rank-sum tests used for comparison between groups and Spearman correlations for correlation analyses. A receiver operating characteristic (ROC) curve analysis was performed to assess diagnostic accuracy. Qualitative data were presented as rates and compared using the Chi-square test. Multivariate analysis was performed using logistic regression analysis. The statistical significance level for all analyses was set at α=0.05.
Results
Participants’ characteristics
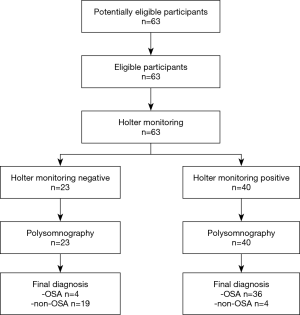
A total of 63 patients, including 52 males and 11 females, completed the PSG and Holter monitoring at the same time. The average age was 47.1±12.7 (range, 20–76) years. The characteristics of participants are presented in Table 1.
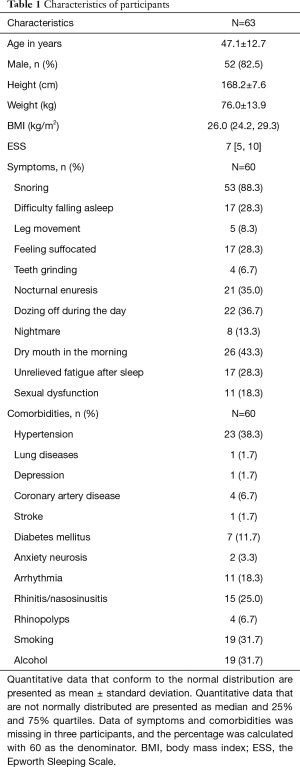
Full table
Parameters of Holter and PSG
There were no adverse events from performing the Holter monitoring or PSG. According to the PSG monitoring results, AHI ≥15 was used as the diagnostic criterion for an OSA diagnosis, with 40 cases, including 38 males and 2 females, meeting this criterion; 23 cases, including 14 males and 9 females, did not meet the OSA diagnosis criterion.
According to the above criterion, patients were divided into two groups: OSA and non-OSA. The total time of apnea events (Ta) and the ratio of the apnea events time to the recording time (Ta/Tm) were the major parameters derived from Holter respiratory waveforms (Figure 1) and were both significantly greater in the OSA group than in the non-OSA group. Conversely, the HRV parameters, SDNN-24 h, SDANN index, rMSSD, pNN50, and HF were all significantly lower in the OSA group than in the non-OSA group (Table 2).
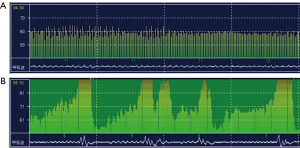
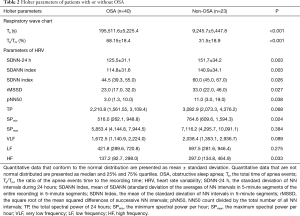
Full table
Correlation analyses revealed that each of the Holter parameters that were found to be significantly different between the OSA and non-OSA groups (Ta, Ta/Tm, SDNN-24 h, SDANN index, rMSSD, pNN50, HF) was correlated with each of the PSG parameters AHI, ODI, SpO2_M, SpO2_L, T<90%, and T<90%/Ts (Table 3). This suggested that Holter-recorded apnea events and HRV indices were highly consistent with PSG-recorded OSA severity and oxygenation impairment.
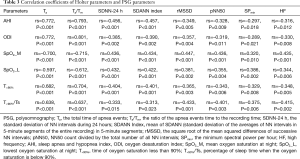
Full table
ROC curve for OSA prediction based on Holter respiratory waveforms
The two main parameters derived from Holter respiratory waveforms, the total time of apnea events and the ratio of the apnea event time to the recording time, were used to predict OSA diagnosis. For an OSA diagnosis based on the total time of apnea events, the corresponding area under the ROC curve was 0.915 (P<0.001, standard error 0.036, 95% CI: 0.845–0.985) (Figure 2A), with 13,817 seconds as the cutoff point, the sensitivity was 90.0%, and the specificity was 82.6%. For an OSA diagnosis based on the ratio of the apnea event time to the recording time, the corresponding area under the ROC curve was 0.921 (P<0.001, standard error 0.035, 95% CI: 0.853–0.988) (Figure 2B), with 47.5% as the cutoff point, the sensitivity was 90.0%, and the specificity was 82.6%. The flow diagram of participants, for an OSA diagnosis based on the ratio of the apnea event time to the recording time, is presented in Figure 3. The flow of participants, for an OSA diagnosis based on the total time of apnea events, is the same as Figure 3. Table 4 shows the accuracy of Holter monitoring for diagnosis of OSA.
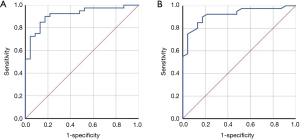

Full table
Logistic regression analysis for OSA prediction based on Holter indices
To set up an algorithm which is based on common HRV indices and clinical characteristics, and could be used conventionally in clinical practice, a logistic regression analysis for OSA prediction based on Holter parameters and clinical characteristics was performed. HRV indices correlated with AHI, including SDNN-24 h, SDANN index, rMSSD, pNN50, SPmin, and HF, as well as clinical characteristics, including BMI and gender, were put into the model. The analysis results show that, gender was the only one parameter independently correlated to OSA diagnosis (P=0.024). And SDNN-24 h, SDANN index, rMSSD, pNN50 were of collinearity (VIF were 67.350, 55.959, 25.747 and 33.407). We conducted logistic regression models with SDNN-24 h and gender, SDANN Index and gender, rMSSD and gender, and pNN50 and gender respectively, and found that SDNN-24 h and gender best predicted OSA (Chi-square =20.359, P<0.001; Table 5), correctly classifying 77.8% of the participants as OSA or non-OSA. The model had a sensitivity of 87.5%, a specificity of 56%, a positive predictive value of 79.5%, and a negative predictive value of 73.7%. According to the logistic regression results, males with an SDNN-24 h ≤177 ms and females with an SDNN-24 h ≤80.9 ms were at high risk of OSA.

Full table
Discussion
Our study found that the Holter-derived respiratory waveforms’ sensitivity for screening OSA is 90.0%, and the specificity is 82.6%; Holter test results of the SDNN-24 h indicate that values ≤177 ms for males and ≤80.9 ms for females predict a higher risk of OSA. Calculations from Holter monitoring and the derived respiratory waveforms can predict the risk of OSA with high sensitivity. SDNN-24 h was common parameter that reported by most commercial Holter monitors. The results of our study provided a convenient way for clinicians to predict OSA risk by Holter monitoring, and improve the diagnosis and treatment rate of OSA.
In our study, the respiratory waveforms derived based on the Holter heart rate trend chart were used to calculate the total time of apnea events and the total time of apnea events as a percentage of sleep time and diagnose OSA. Our results show that compared to the PSG diagnostic method, using AHI ≥15 as the intercept point, Holter’s sensitivity is 90.0% and specificity is 82.6% for screening OSA. This result suggested a high sensitivity. Most patients with a high risk of OSA could be distinguished by Holter. Similar results were reported by Lyons et al., who calculated the respiratory power index using common ECG data, combined with clinical indicators such as BMI, and predicted a sensitivity of 91.7% and a specificity of 27.3% for severe OSA (AHI ≥30/h) (11).
ECG signals are closely related to respiration. The autonomic nervous system is responsible for regulating breathing and heart rate, among other processes, and the two divisions of the autonomic nervous system (sympathetic and parasympathetic) can work in seemingly opposite yet complementary ways to increase or decrease breathing and heart rate based on contextual and environmental demands. The system works in such a way that changes or adjustments in the breathing cycle causes the heart rate to change. This relationship can be illustrated by changes in the ECG signal. Accompanied by respiratory relaxation of the thorax and changes in lung air content, the transthoracic electrical impedance changes accordingly (12). These changes cause the QRS amplitude of the recorded ECG to fluctuate up and down, that is, the regular deviation of the average ECG axis direction. According to the regular deviation of the average ECG axis direction, algorithms can be used to derive the corresponding respiratory waveforms and to calculate the apnea/hypopnea events.
A growing number of studies show that OSA has a high prevalence along with many cardiovascular diseases, for example, 34% of patients with hypertension (3), 21–74% of patients with atrial fibrillation (4), and 46% of patients with myocardial infarction also have a diagnosis of OSA (5), Some patients with OSA notice cardiovascular symptoms such as chest tightness, palpitations, nocturnal angina pectoris, nocturnal arrhythmia, and refractory hypertension; thus, they often first consult the department of cardiology (13), which may delay or conceal the diagnosis of OSA. Due to the wide use of Holter and the high prevalence of OSA in many cardiovascular diseases, if Holter technicians could report the risk of OSA in addition to the traditional Holter report, the diagnosis rate of OSA would be improved significantly. It is more suitable for finding out early warning signs or screening of OSA during routine Holter examination in primary medical units. Active detection of OSA in patients is important for clinical practice. For OSA-positive patients based on the Holter screening, PSG or HST can be further performed to confirm the diagnosis and determine the severity of the disease.
Besides respiratory waveforms, HRV indices were the other important parameters provided by Holter. Chikao Nakayama developed an apnea/normal breathing recognition model based on HRV, which had a sensitivity of 76% for screening OSA (AHI ≥15) (14). Moreover, Holter HRV indices are correlated with the severity of OSA. Previous studies have shown that SDNN, pNN50, and other time domain indicators decrease, and LF/HF increases in patients with OSA (15). Patients with OSA with AHI ≥30 have significant changes in time and frequency indicators compared with patients without OSA. After 3 months of continuous positive airway pressure treatment, HRV indices can recover to a certain extent (16). Although HRV indices are closely related to OSA severity and could be provided by traditional Holter reports, nowadays, Holter technicians find it difficult to judge the risk of OSA based on the HRV indices (16). Because of that, there are tens of HRV indices, but no clear cutoff points to distinguish OSA and non-OSA. Moreover, there is no equation based on these HRV indices to calculate the risk of OSA (16).
The results of this study show that SDNN-24 h and gender can predict the risk of OSA. The sensitivity of SDNN-24 h combined with gender for predicting moderate to severe OSA is 87.5%, which can accurately distinguish the risk of OSA in 77.8% of patients. According to the logistic regression model, Holter test results of the SDNN-24 h indicate that values ≤177 ms for males and ≤80.9 ms for females predict a higher risk of OSA. SDNN-24 h is the standard reporting information of the Holter monitor; therefore, no special software or procedures are needed to run the monitor and obtain the necessary/useful information, making it well suited to be widely used in clinical practice. If Holter technicians can assess the risk of OSA disease based on the patient’s gender and SDSN-24 h results, include the information on OSA risk in their reports, and suggest a follow-up to assess OSA, missing the diagnosis or misdiagnosis of OSA can be significantly decreased.
The limitations of this study were that the sample size was small, the participants were mostly male and that the participants were only the patients who visited the sleep center. Holter examination is mostly performed in patients with cardiovascular symptoms such as chest tightness and palpitations (17). Further research studies are needed to determine whether Holter is appropriate for OSA prediction in patients with cardiovascular symptoms, arrhythmia, coronary heart disease, and heart failure.
Acknowledgments
The authors gratefully thank Yunlian Xue from the Statistics Office, Information and Statistics Center, Guangdong Provincial People’s Hospital (Guangdong Academy of Medical Sciences) for statistical assistance in this work.
Funding: This work was supported by the National Natural Science Foundation of China (Grant No. 81870077) to QO.
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at http://dx.doi.org/10.21037/jtd-20-3078
Data Sharing Statement: Available at http://dx.doi.org/10.21037/jtd-20-3078
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd-20-3078). Dr. QO reports grants from the National Natural Science Foundation of China, during the conduct of the study. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from all study patients. Ethics approval for this study was obtained from the ethics committee of Guangdong Provincial People’s Hospital (No. GDREC2019757H).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kapur VK, Auckley DH, Chowdhuri S, et al. Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: an American Academy of Sleep Medicine Clinical Practice Guideline. J Clin Sleep Med 2017;13:479-504. [Crossref] [PubMed]
- Costa LE, Uchôa CH, Harmon RR, et al. Potential underdiagnosis of obstructive sleep apnoea in the cardiology outpatient setting. Heart 2015;101:1288-92. [Crossref] [PubMed]
- Bleckwenn M, Linnenkamp D, Weckbecker K, et al. Prevalence of sleep apnea in patients with first diagnosis of hypertension. MMW Fortschr Med 2019;161:3-6. [Crossref] [PubMed]
- Linz D, McEvoy RD, Cowie MR, et al. Associations of obstructive sleep apnea with atrial fibrillation and continuous positive airway pressure treatment: a review. JAMA Cardiol 2018;3:532-40. [Crossref] [PubMed]
- Tan LL, Ting J, Balakrishnan I, et al. Sleep apnea evolution and left ventricular recovery after percutaneous coronary intervention for myocardial infarction. J Clin Sleep Med 2018;14:1773-81. [Crossref] [PubMed]
- Kim J, Keenan BT, Lim DC, et al. Symptom-based subgroups of Koreans with obstructive sleep apnea. J Clin Sleep Med 2018;14:437-43. [Crossref] [PubMed]
- Magnusdottir S, Hilmisson H. Ambulatory screening tool for sleep apnea: analyzing a single-lead electrocardiogram signal (ECG). Sleep Breath 2018;22:421-9. [Crossref] [PubMed]
- Mueller A, Fietze I, Voelker R, et al. Screening for sleep-related breathing disorders by transthoracic impedance recording integrated into a Holter ECG system. J Sleep Res 2006;15:455-62. [Crossref] [PubMed]
- Berry RB, Budhiraja R, Gottlieb DJ, et al. Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med 2012;8:597-619. [Crossref] [PubMed]
- Kushida CA, Littner MR, Morgenthaler T, et al. Practice parameters for the indications for polysomnography and related procedures: an update for 2005. Sleep 2005;28:499-521. [Crossref] [PubMed]
- Lyons MM, Kraemer JF, Dhingra R, et al. Screening for Obstructive Sleep Apnea in Commercial Drivers Using EKG-Derived Respiratory Power Index. J Clin Sleep Med 2019;15:23-32. [Crossref] [PubMed]
- Sharma H, Sharma KK. ECG-derived respiration based on iterated Hilbert transform and Hilbert vibration decomposition. Australas Phys Eng Sci Med 2018;41:429-43. [Crossref] [PubMed]
- Hou H, Zhao Y, Yu W, et al. Association of obstructive sleep apnea with hypertension: a systematic review and meta-analysis. J Glob Health 2018;8:010405 [Crossref] [PubMed]
- Nakayama C, Fujiwara K, Sumi Y, et al. Obstructive sleep apnea screening by heart rate variability-based apnea/normal respiration discriminant model. Physiol Meas 2019;40:125001 [Crossref] [PubMed]
- Urbanik D, Gać P, Martynowicz H, et al. Obstructive sleep apnea as a predictor of reduced heart rate variability. Sleep Med 2019;54:8-15. [Crossref] [PubMed]
- Nastałek P, Bochenek G, Kania A, et al. Heart rate variability in the diagnostics and CPAP treatment of obstructive sleep apnea. Adv Exp Med Biol 2019;1176:25-33. [Crossref] [PubMed]
- Mubarik A, Iqbal AM. Holter monitor. In: StatPearls [Internet]. Treasure Island: StatPearls Publishing, 2020.


