Endobronchial closure of bronchopleural fistula using Amplatzer device
Introduction
Bronchopleural fistula (BPF) is an uncommon, but severe complication of lobectomy and pneumonectomy and is associated with high morbidity and mortality rates (1,2). The incidence of BPF has been significantly reduced in the last decades, but still represents a serious complication. The incidence is significantly higher after pneumonectomy compared to lobectomy and ranges between 0.5% and 5.9% (3). The mortality rate associated with BPF is alarmingly high and ranges between 16% and 71% (2,4). Differences in the incidence of BPF have been described according to the side of surgical procedure: higher incidence has been reported for right-sided pneumonectomy and for right lobectomy of the lower lobe (2,3,5). Moreover, patients with malignant conditions show significantly higher rates of BPF than patients with benign disease (1).
Surgical treatment options for BPF include different techniques from chest tubes to muscle flaps and thoracoplasty. Endoscopic options include biologic glue (e.g., fibrin, collagen) which is injected in the surrounding mucosa as well as covered stents or coils (2,5-7). Treatment success is variable and depends on the size of the fistula and the underlying disease (7-9).
In recent years, different reports about a novel method of BPF closure have been published in the literature. Amplatzer devices (ADs), especially Amplatzer septal occluders (ASO) which have been developed for the transcatheter closure of cardiac atrial septum defects, have been successfully used for the bronchoscopic occlusion of BPF (9-11). In this study, we describe the successful treatment of three patients with BPF after surgical resection of non-small cell lung cancer (NSCLC) by lobectomy or pneumonectomy with this new endoscopic method.
Materials and methods
The AD (ASO; St. Jude Medical, Plymouth, Minnesota, United States) is a self-expandable, double-disk device designed for the occlusion of atrial septal defects. The device is made of braided nitinol and interwoven polyester to promote occlusion and tissue in-growth. Due to the properties of nitinol, the device is compressible inside a catheter and returns into its shape when deployed in the BPF to occlude the defect by a self-centering waist. The device can be easily recaptured and redeployed for optimal placement. The AD is available in diameters ranging from 4 to 38 mm. Because of the large range of sizes of the AD, the device can be perfectly matched to the diameter of fistula size.
All three patients were informed about the planned intervention and the off-label use of AD for the occlusion of BPF. Written informed consent was obtained from all patients before the procedure.
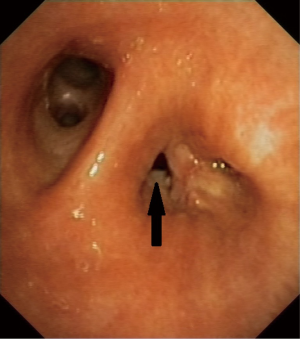
The bronchoscopic placement of the device was performed using combined rigid-flexible bronchoscopy. General anaesthesia was established, a rigid bronchoscope was introduced into the trachea and jet ventilation was initiated. A flexible bronchoscope (Type BF 1T-180; Olympus, Hamburg, Germany) was introduced and the BPF was located in the bronchial stump (Figure 1). Using bronchography with contrast agent application, the anatomy and size of the fistula was analyzed. The size of AD was calculated using computed tomography and direct measurement during bronchoscopy. The size of AD was chosen according to the size of the fistula and the diameter of the bronchial stump, so that the device completely covered the fistula and also fit into the stump (Figures 2,3). The delivery catheter with the loaded device was introduced through the rigid bronchoscope and the device was deployed into the BPF under direct endoscopic vision and fluoroscopic guidance. Once placed correctly, the device was released by unscrewing the attached cable. To verify the complete occlusion of the BPF, a second bronchography with contrast agent application was performed (Figure 4). After removal of the bronchoscope, the patient was transferred into the recovery room and a standard X-ray was performed.
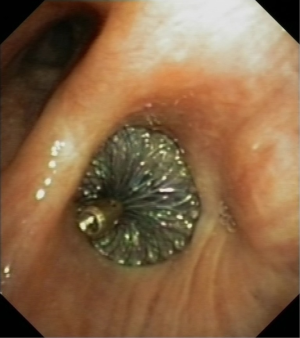
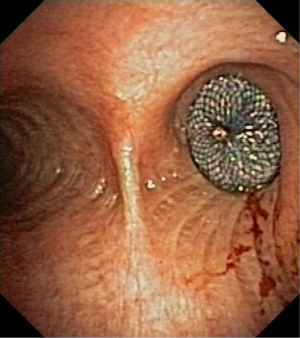
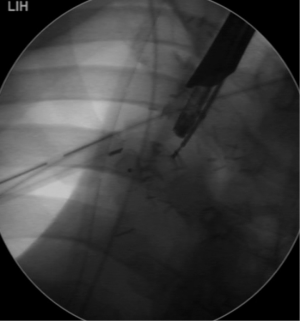
Results
Three male patients with BPF were treated at our institution between July and November 2013. Mean time from operation to appearance of bronchial stump insufficiency was 2.3 months (range, 2-3 months). All patients developed BPF after surgery for lung cancer. Two patients underwent lobectomy of the right lower lobe and one patient had right pneumonectomy. Patient characteristics and treatment data for the three patients are displayed in Table 1.
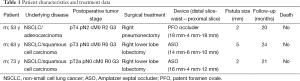
Full table
Preceding conservative and operative treatment attempts had been unsuccessful in all three patients. Closure of the fistula after lobectomy had been tried with instillation of fibrin glue in one patient. Another patient underwent partial thoracoplasty with latissimus dorsi flap for fistula closure, but no sufficient closure was achieved. For the patient after pneumonectomy, no definite surgical treatment was possible because of empyema and very poor general condition. Therefore, thoracoscopy had been performed with irrigation of the thoracic cavity and placement of two chest tubes. Due to a reduced clinical condition, closure of the fistula with fibrin glue had been tried, but unfortunately no stability of the fibrin glue in the BFP was achieved.
The two patients with BPF after lobectomy of the right lower lobe were treated with bronchoscopic ASO implantation St. Jude Medical, Plymouth, Minnesota, United States). BPF after pneumonectomy was treated with implantation of Amplatzer patent foramen ovale (PFO) occluder (PFO; St. Jude Medical, Plymouth, Minnesota, United States). Size of the AD was calculated using computed tomography and endoscopic measurements. There were no immediate complications related to the procedure and no patient needed mechanical ventilation after bronchoscopy. There was no 30-day-mortality and success rate was 100%. All three patients had immediately benefited from the procedure. A marked improvement in the general condition and a decrease of infection parameters in the blood tests was shown in the first days after the implantation of AD. Chest tubes were removed in the further clinical course.
The mean follow-up was 22 months (range, 20-24 months). Computed tomography of the thoracic cavity and flexible bronchoscopy during the follow-up period showed the correct position of AD and local granulation tissue around the device. Consequently, a late dislocation of the AD surrounded by granulation tissue is hardly possible. Actually, the three patients are alive and in a good general condition without recurrence of the BPF. No stenosis and no further infection occurred in the thoracic cavity.
Discussion
BPF occurring after lobectomy or pneumonectomy have to be regarded as a severe complication and are associated with high morbidity and mortality rates. Risk factors for the development of a bronchial stump insufficiency include an underlying malignant disease, previous chemotherapy or radiotherapy, carcinoma infiltration of the resection margin and operation of the right side (2,3). Another risk factor for BPF might be a poor differentiated carcinoma as it is seen in our patients. Various surgical and medical options for the closure of these fistulas are described in the literature, but the management is still difficult because no reliable treatment modality is available (6,7,12). For a long time, endoscopic techniques have been considered for small BPF, mostly for high-risk patients without the option for surgical treatment. Biological glue, coils or covered stents are mainly used in these cases (13-15). Larger BPF could not be treated by endoscopic techniques because neither coils nor fibrin glue was suitable for large bronchial stump fistulas due to insufficient stability in the lesion. In recent years, a novel minimally invasive method for the treatment of BPF with ASOs has been utilized and described in a few reports (9,16). Various applications of these devices have been reported previously, including coronary fistula and arteriovenous malformation (9,17). Therefore, these devices are produced to achieve an effective closure of the defect and can be repositioned for perfect placement (17).
We describe our experience with the novel method using AD for the occlusion of BPF after lobectomy and pneumonectomy for lung cancer. In these cases, previous conservative and operative treatment modalities had been unsuccessful. A successful closure of the fistula resulted in the disappearance of the BPF-related symptoms. After three to 6 months, bronchoscopic inspection showed a sufficient granulation tissue around the implanted device. For this reason, a slippage is no longer possible in the further course.
Possibly, the closure of BPF using AD is a safe alternative to surgery for patients with a significantly reduced general condition and with the dreaded complication of an insufficiency of the bronchial stump. Hereby, improvement of general condition can rapidly be achieved in the postinterventional course. Maybe, the closure of BPF with AD represents a safe alternative to surgical intervention for this dreaded complication in future times because AD are available for the closure of different fistula sizes which cannot be treated successfully with conservative treatment modalities (9). As we describe, even the closure of a BPF after lobectomy or pneumonectomy is feasible when surgical treatment possibilities are not effective or not feasible because of reduced general condition.
Conclusions
Based on our clinical experience, we could underline the existing data for the use of AD as a safe and effective tool for the endoscopic closure of larger BPF following lobectomy or pneumonectomy for patients in poor general condition without a surgical treatment option. Especially for patients after radical operation for lung cancer, followed by BPF with empyema or pneumonia, this safe, sufficient and minimally-invasive treatment option can be lifesaving.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Cerfolio RJ. The incidence, etiology, and prevention of postresectional bronchopleural fistula. Semin Thorac Cardiovasc Surg 2001;13:3-7. [PubMed]
- Sirbu H, Busch T, Aleksic I, et al. Bronchopleural fistula in the surgery of non-small cell lung cancer: incidence, risk factors, and management. Ann Thorac Cardiovasc Surg 2001;7:330-6. [PubMed]
- Hatz RA, Klotz LV. Consequences of pneumonectomy in the early and late phases Chirurg 2013;84:497-501. [PubMed]
- Farkas EA, Detterbeck FC. Airway complications after pulmonary resection. Thorac Surg Clin 2006;16:243-51. [PubMed]
- Boudaya MS, Smadhi H, Zribi H, et al. Conservative management of postoperative bronchopleural fistulas. J Thorac Cardiovasc Surg 2013;146:575-9. [PubMed]
- Lang-Lazdunski L. Closure of a bronchopleural fistula after extended right pneumonectomy after induction chemotherapy with BioGlue surgical adhesive. J Thorac Cardiovasc Surg 2006;132:1497-8. [PubMed]
- Tao H, Araki M, Sato T, et al. Bronchoscopic treatment of postpneumonectomy bronchopleural fistula with a collagen screw plug. J Thorac Cardiovasc Surg 2006;132:99-104. [PubMed]
- Ranu H, Gatheral T, Sheth A, et al. Successful endobronchial seal of surgical bronchopleural fistulas using BioGlue. Ann Thorac Surg 2009;88:1691-2. [PubMed]
- Fruchter O, Kramer MR, Dagan T, et al. Endobronchial closure of bronchopleural fistulae using amplatzer devices: our experience and literature review. Chest 2011;139:682-7. [PubMed]
- Fruchter O, El Raouf BA, Abdel-Rahman N, et al. Efficacy of bronchoscopic closure of a bronchopleural fistula with amplatzer devices: long-term follow-up. Respiration 2014;87:227-33. [PubMed]
- Kramer MR, Peled N, Shitrit D, et al. Use of Amplatzer device for endobronchial closure of bronchopleural fistulas. Chest 2008;133:1481-4. [PubMed]
- Puskas JD, Mathisen DJ, Grillo HC, et al. Treatment strategies for bronchopleural fistula. J Thorac Cardiovasc Surg 1995;109:989-95; discussion 995-6. [PubMed]
- Lois M, Noppen M. Bronchopleural fistulas: an overview of the problem with special focus on endoscopic management. Chest 2005;128:3955-65. [PubMed]
- Paul S, Talbot SG, Carty M, et al. Bronchopleural fistula repair during Clagett closure utilizing a collagen matrix plug. Ann Thorac Surg 2007;83:1519-21. [PubMed]
- Dutau H, Breen DP, Gomez C, et al. The integrated place of tracheobronchial stents in the multidisciplinary management of large post-pneumonectomy fistulas: our experience using a novel customised conical self-expandable metallic stent. Eur J Cardiothorac Surg 2011;39:185-9. [PubMed]
- Fruchter O, Bruckheimer E, Raviv Y, et al. Endobronchial closure of bronchopleural fistulas with Amplatzer vascular plug. Eur J Cardiothorac Surg 2012;41:46-9. [PubMed]
- Hart JL, Aldin Z, Braude P, et al. Embolization of pulmonary arteriovenous malformations using the Amplatzer vascular plug: successful treatment of 69 consecutive patients. Eur Radiol 2010;20:2663-70. [PubMed]