Randomized controlled trials of induction treatment and surgery versus combined chemotherapy and radiotherapy in stages IIIA-N2 NSCLC: a systematic review and meta-analysis
Introduction
Lung cancer is the leading cause of cancer-related deaths. Worldwide, roughly 1.5 million new cases of lung cancer are diagnosed annually (1) with about 85% being non-small-cell lung cancers (NSCLCs) (2). For stage IIIA-N2 NSCLC, which is defined as involvement of the ipsilateral mediastinal or subcarinal lymph nodes (3), neoadjuvant chemotherapy followed by surgery has been shown to lengthen survival in selected patients with stage IIIA NSCLC (4-7). Also, combined chemotherapy and radiotherapy has been proven that it can improve the outcome for these patients compared with radiotherapy alone (5,8-12). Whereas, there remains discussion whether induction treatment followed by surgery is the best option compared with combined chemoradiotherapy for stage IIIA-N2 NSCLC.
Examination and synthesis of the limited available data comparing induction treatment plus surgery and combined chemoradiotherapy may allow physicians to determine the optimal treatment for patients with IIIA-N2 disease. Recently, three large phase III randomized clinical trials (RCTs) was published their results to evaluate the survival benefit of induction treatment plus surgery compared with combined chemoradiotherapy (13-15). However, the efficacy of preoperative induction treatment in improving postoperative survival in patients with stages IIIA-N2 NSCLC remains controversial. We therefore conducted a systematic review and meta-analysis of the published phase III RCTs to quantitatively evaluate survival benefit of patients who underwent these two kinds of treatments.
Materials and methods
Eligibility criteria
RCTs comparing preoperative induction treatment plus surgery with combined chemoradiotherapy were conducted using the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) standards (16) as the basis for reporting the materials and methods of this study, and aimed to include the patients with stages IIIA-pN2 NSCLC if they started after Jan 1, 1980. The following criteria for eligibility into this meta-analysis were set before collecting the articles: (I) the trials had to be phase III RCTs comparing the survival between a groups receiving preoperative induction treatment plus surgery and another group receiving combined chemoradiotherapy; (II) the studies involved patients with stages IIIA-pN2 NSCLC based upon international staging criteria (17); (III) the hazard ratios (HRs) and confidence intervals (CIs) of the patients who underwent preoperative induction treatment plus surgery and those who received combined chemoradiotherapy could be calculated at specified time intervals after surgery from the survival rates in the article; (IV) the median follow-up time of the study exceeded at least 2 years; (V) published and unpublished trials were sought, with no language restriction, using randomised trial search filters for PubMed, EMBASE and Cochrane library.
Data collection
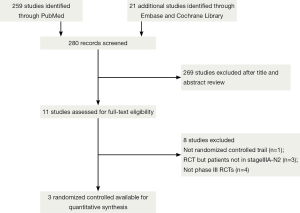
Two investigators independently searched eligible trials, and discrepancies were resolved by discussion between them. Non-English publications were evaluated based on their English abstract and the translation of their main text. The keywords “IIIA-N2 Non-small-cell lung cancer + chemotherapy plus radiotherapy”, “IIIA-N2 NSCLC + radiotherapy plus chemotherapy with or without surgical resection”, totally hit 280 citations. The relevant clinical studies were manually selected based on summary analyses. Articles reporting studies unrelated to our question were excluded, and finally only three studies (13-15) were found to fulfill all of our eligibility criteria (Table 1).
Full table
Validity assessment
We conducted the validity assessment referred to the meta-analysis performed by Wright et al. (18). Two reviewers evaluated the quality of the studies independently with disagreements resolved by consensus. Using the Cochrane approach to allocation concealment, trials were described as having adequate, unclear, or inadequate concealment (19). The reviewers assessed whether there was blinding of outcome assessment and adequate description of with-drawls (20). The adequacy of the method of randomization was also assessed as described by Jadad et al. (20). Finally, an assessment was made as to whether the trial results used intention to treat analysis (21,22). The authors of included studies were asked to verify assessments of study methodology where possible.
Statistical analyses
Statistical analyses for the meta-analysis were performed with RevMan (Review Manager Version 5.3 for Windows, Cochrane Collaboration. Oxford, UK, 2014), and a pooled relative risk was calculated with 95% CIs. The methods described by Parmar et al. were used to estimate the HRs and variance indirectly from CIs or P values for the log rank test (23). To undertake a random effects meta-analysis, the standard errors of the study-specific estimates are adjusted to incorporate a measure of the extent of variation, or heterogeneity, among the treatment effects observed in different studies. The survival rates were derived from the published survival curves when not provided explicitly in the text or tables. Data extraction from the survival curves was done by two researchers independently, and the mean measured values were used for the analysis. Heterogeneity was evaluated with the χ2 distribution test with rejection region equal to 0.1; and the I2 test whereby I2=0% indicated no heterogeneity, I2=0-40% indicated low heterogeneity, I2=40-60% indicated moderate heterogeneity, and I2=50-90% indicated high heterogeneity, I2=75-100% indicated maximum heterogeneity (19).
Results
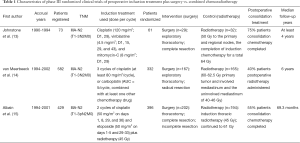
Three randomized phase III trials, with a total of 1,084 patients, were included for survival analysis (Figure 1). The trial characteristics and the treatment schedules used are listed in Table 1. The two studies reported by Johnstone et al. (13) and van Meerbeeck et al. (14) merely used the platinum based regimen for preoperative induction treatment, which differs from the trial reported by Albain et al. (15) received the platinum based regimen plus radiotherapy (45 Gy) for preoperative induction treatment. After induction treatment was completed, patients registered who were response to the induction treatment can be randomized into next step. Thus, the results were based on three randomized controlled trials (789 patients), and then preoperative induction treatment followed by surgery was assigned to a total of 398 patients, while combined chemotherapy and radiotherapy without surgery was assigned to 391 patients. The overall classification of histologic types was 283 (36.9%) squamous cell carcinomas (surgery: non-surgery =137:146), 291 (36.9%) adenocarcinomas (153:138, respectively), 146 (18.5%) large cell carcinomas (73:73, respectively), 69 (8.7%) miscellaneous types (35:34, respectively). There was no clear evidence of a difference in the effect on survival by chemotherapy regimen or scheduling, number of drugs, platinum agent used, or whether postoperative chemo or radiotherapy was given. There was no clear evidence that particular types of patient defined by age, sex, performance status, histology, or clinical stage benefited more or less from both of them.
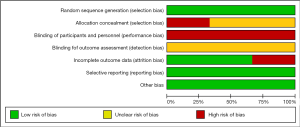
The quality of included trials was shown (Table 2). Intergroup trial 0139 (15) was found to be inadequate in allocation concealment. All of the included studies contained a clear statement that they had conducted method of randomization and intention to treat analysis. Further quality details of the trials are shown in the Table 2. According to the three trials’ methodological quality, we reviewed authors’ judgements about each risk of bias item presented as figure (Figure 2).

Full table
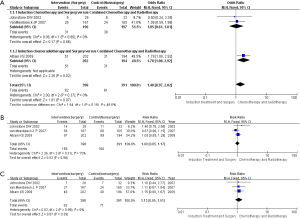
For the whole groups, there was no improvement in 2-year overall survival (OS) [risk ratio (RR) =1.00; 95% CI, 0.85-1.17; P=0.98] and 4-year OS (RR =1.13; 95% CI, 0.85-1.51; P=0.39) compared with the combined chemoradiotherapy arm (Figure 3A,B). From the subgroup analysis, when we performed a meta-analysis on the two studies reported by Johnstone et al. (13) and van Meerbeeck et al. (14), the pooling data from both studies (n=393) indicated that there was no significant difference in 3 years progression free survival (PFS) (RR =1.05; 95% CI, 0.61-1.81; P=0.86) (Figure 3C) regarded the preoperative chemotherapy as induction treatment. However, according to the sub-meta-analysis on the study reported by Albain et al. (15), it showed a significant PFS between the intervention arm and control arm (RR =1.78; 95% CI, 1.08-2.92; P=0.02) (Figure 3C). Thus, when the radiotherapy was added into preoperative induction treatment, compared with only preoperative chemotherapy as induction treatment, it could improve PFS. Heterogeneity testing indicated that the study at 3 years PFS was low heterogeneity [I2=0%; P for χ2 test =0.37 (>0.1)] and at 2 years OS and 4 years OS were also low heterogeneity [I2=15%; P for χ2 test =0.31 (>0.1); I2=0%; P for χ2 test =0.99 (>0.1)], respectively.
Discussion
In many centers, they have demonstrated that stages IIIA-N2 NSCLC patients have significant survival advantage benefited from preoperative induction treatment plus surgery compared with surgery or radiotherapy alone (4-7,24). In addition, it has been proven that combined chemoradiotherapy can improve IIIA-N2 NSCLC patients OS compared with radiotherapy or surgery alone (5,8-12). The meta-analysis reported by Wright et al. (18) tried to compare chemotherapy followed by surgery with sequential chemotherapy and radiotherapy, but it was inconclusive because of small numbers. Given the failure of numerous clinical trials attempting to answer this question as well as the small sample sizes of individual published studies on this topic, we attempted to evaluate and synthesize the available data to provide clinicians with summarized evidence-based information to guide them in taking care of patients with stage IIIA (N2) disease. Thus, we integrated the three large randomized trials with a total of 1,084 patients to analyze and compare which one is the optimal treatment.
Our meta-analysis pointed out that whether preoperative induction treatment plus surgery or combined chemoradiotherapy was given, it showed no improvement in the stages IIIA-N2 NSCLC patients 2-year OS (RR =1.00; 95% CI, 0.85-1.17; P=0.98) and 4-year OS (RR =1.13; 95% CI, 0.85-1.51; P=0.39). It matches with the finding from the results reported by the three trials (13-15) showed no significant OS benefit in treatment between preoperative induction treatment plus surgery and chemoradiotherapy. This implies that patients in stages IIIA-N2 NSCLC might be received either of the two treatments, and their OS could be no significant difference.
However, although the OS have been demonstrated without any difference between the two treatments, it still need us to make clear its appropriate context of patients with unresectable IIIA-N2 disease what the crucial term “unresectable” was clearly defined in the article. And another concern is how the assessment of irresectability was performed (25). Because there have different European series who published their 5-year survival rates of 36% (Swiss group) (26), 34% (Essen group) (27), or 30% (Leuven group) (28) among patients with IIIA-N2 disease but resect after induction treatment. The rationale of these studies is to provide surgery as the best local treatment for resectable NSCLC and improve outcome by induction therapy to manage distant micrometastasis. Though the result of our meta-analysis emphasized the OS without significance between the two treatments, this should not lead to the over-interpretation that combined chemoradiotherapy is the best choice for every patient with IIIA-N2 NSCLC. We also concur with the editorial that patients who are good candidates for surgery may still be appropriately managed by using resection rather than radiation (29).
From the subgroup analysis, when the induction treatment was separated into preoperative chemoradiotherapy and preoperative chemotherapy treatment, and then compared them with combined chemoradiotherapy, it showed some potential advantages for the patients PFS when radiation therapy was added into preoperative induction treatment. The 3 years PFS synthesized by the two studies (13,14) was no significant difference (RR =1.05; 95% CI, 0.61-1.81; P=0.86), and it matches with the results reported by Johnstone et al. (13) and van Meerbeeck et al. (14) (HR =1.06; 95% CI, 0.85-1.33; P=0.605). Nevertheless, we compared the data reported by the Intergroup trial 0139 (15), and it showed a significant PFS (RR =1.78; 95% CI, 1.08-2.92; P=0.02), which indirectly suggests that induction chemoradiotherapy as a preoperative induction treatment might be superior to induction chemotherapy without radiotherapy. The study WJTOG9903 (30) also tried to ascertain whether induction-concurrent radiotherapy added to chemotherapy could improve the survival of patients undergoing surgery for stage IIIA N2 NSCLC. Although PFS had not been improved in the chemoradiotherapy plus surgery arm vs. the chemotherapy plus surgery arm (median, 12.4 vs. 9.7 months; HR =0.68; 95% CI, 0.38-1.21; P=0.187), Katakami (30) pointed out that these differences are not statistically significant due to the small sample size. And they demonstrated that the addition of radiotherapy to the induction chemotherapy regimen for stage IIIA (N2) NSCLC appears to confer better local control without adding significant adverse events, and tumor down-staging after induction therapy is an important factor for improving patient survival. The retrospective review published by Martin et al. (31), the retrospective study conducted by Darling et al. (32) and the results published by Higgins et al. (33) also supported potential advantages of an increased pathologic complete response rate and improved local control for adding the radiation into induction therapy, and then improved the PFS.
Although the systematic review and meta-analysis performed by Shah et al. (34) to compare induction chemoradiotherapy vs. induction chemotherapy alone demonstrated that published evidence is limited but does not support the inclusion of radiation therapy in induction regimens for stage IIIA (N2) NSCLC, it did not affect our result. Because their meta-analysis indeed lacked sufficient data, such as WJTOG9903 (30) and the retrospective study conducted by Darling et al. (32), and their meta-analysis, with a total of seven studies, included some low qualified studies and the statistical heterogeneity were deemed imprecise. In addition, we failed to compare the administration of postoperative radiotherapy in the EORTC (14) with the consolidation chemotherapy in the IG trial 0139 (15), which might result in an imbalance of better local control and better PFS in surgery arm of the intergroup trial, but some published studies (35,36) proved that the locoregional relapse rate was higher in the postoperative radiotherapy arm.
The heterogeneity test detected low heterogeneity between the combined studies. The included studies were considered low heterogeneity for the following reasons. Firstly, the ratio of overall classification of histologic types in each study is closed to 1:1, and the ratio of gender in each study is almost equal (13-15). Secondly, three studies included only clinical stage IIIA-N2 NSCLC patients. And the therapeutic regimens were also similar among the studies. As for the Intergroup Trial 0139, radiation therapy was added into preoperative induction treatment, but the subgroup analysis has been given, which turns out to be still very low heterogeneity. Thirdly, all the trials are phase III RCTs and have enough follow-up time. In addition, according to the three trials’ methodological quality, we reviewed authors’ judgements and figured out each risk of bias item, and it also indicated without serious problem to affect our meta-analysis.
Conclusions
There was no significant OS benefit of induction treatment plus surgery compared with combined chemoradiotherapy in patients with NSCLC (stages IIIA-N2) at 2 and 4 years. However, from the subgroup analysis, we could conclude PFS could be improved when radiation therapy was added into preoperative induction treatment. Given the potential advantages of adding radiation preoperatively, clinicians should consider using this treatment strategy in the stage IIIA-N2 disease after fully assessment of the patients.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011;61:69-90. [PubMed]
- American Cancer Society. Cancer facts and figures 2007. Atlanta: American Cancer Society, 2007.
- Goldstraw P, Crowley J, Chansky K, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol 2007;2:706-14. [PubMed]
- Rosell R, Gómez-Codina J, Camps C, et al. A randomized trial comparing preoperative chemotherapy plus surgery with surgery alone in patients with non-small-cell lung cancer. N Engl J Med 1994;330:153-8. [PubMed]
- Roth JA, Fossella F, Komaki R, et al. A randomized trial comparing perioperative chemotherapy and surgery with surgery alone in resectable stage IIIA non-small-cell lung cancer. J Natl Cancer Inst 1994;86:673-80. [PubMed]
- Johnson DH, Einhorn LH, Bartolucci A, et al. Thoracic radiotherapy does not prolong survival in patients with locally advanced, unresectable non-small cell lung cancer. Ann Intern Med 1990;113:33-8. [PubMed]
- Ettinger DS, Akerley W, Bepler G, et al. Non-small cell lung cancer. J Natl Compr Canc Netw 2010;8:740-801. [PubMed]
- Marino P, Preatoni A, Cantoni A. Randomized trials of radiotherapy alone versus combined chemotherapy and radiotherapy in stages IIIa and IIIb nonsmall cell lung cancer. A meta-analysis. Cancer 1995;76:593-601. [PubMed]
- Trovò MG, Minatel E, Veronesi A, et al. Combined radiotherapy and chemotherapy versus radiotherapy alone in locally advanced epidermoid bronchogenic carcinoma. A randomized study. Cancer 1990;65:400-4. [PubMed]
- Pritchard RS, Anthony SP. Chemotherapy plus radiotherapy compared with radiotherapy alone in the treatment of locally advanced, unresectable, non-small-cell lung cancer. A meta-analysis. Ann Intern Med 1996;125:723-9. [PubMed]
- Perez CA, Pajak TF, Rubin P, et al. Long-term observations of the patterns of failure in patients with unresectable non-oat cell carcinoma of the lung treated with definitive radiotherapy. Report by the Radiation Therapy Oncology Group. Cancer 1987;59:1874-81. [PubMed]
- Elias AD, Kumar P, Herndon J 3rd, et al. Radiotherapy versus chemotherapy plus radiotherapy in surgically treated IIIA N2 non-small-cell lung cancer. Clin Lung Cancer 2002;4:95-103. [PubMed]
- Johnstone DW, Byhardt RW, Ettinger D, et al. Phase III study comparing chemotherapy and radiotherapy with preoperative chemotherapy and surgical resection in patients with non-small-cell lung cancer with spread to mediastinal lymph nodes (N2); final report of RTOG 89-01. Radiation Therapy Oncology Group. Int J Radiat Oncol Biol Phys 2002;54:365-9. [PubMed]
- van Meerbeeck JP, Kramer GW, Van Schil PE, et al. Randomized controlled trial of resection versus radiotherapy after induction chemotherapy in stage IIIA-N2 non-small-cell lung cancer. J Natl Cancer Inst 2007;99:442-50. [PubMed]
- Albain KS, Swann RS, Rusch VW, et al. Radiotherapy plus chemotherapy with or without surgical resection for stage III non-small-cell lung cancer: a phase III randomised controlled trial. Lancet 2009;374:379-86. [PubMed]
- Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol 2009;62:e1-34. [PubMed]
- Rami-Porta R, Crowley JJ, Goldstraw P. The revised TNM staging system for lung cancer. Ann Thorac Cardiovasc Surg 2009;15:4-9. [PubMed]
- Wright G, Manser RL, Byrnes G, et al. Surgery for non-small cell lung cancer: systematic review and meta-analysis of randomised controlled trials. Thorax 2006;61:597-603. [PubMed]
- Higgins JP, Green S. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0. The Cochrane Collaboration, 2011. Available online: http://handbook.cochrane.org/
- Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996;17:1-12. [PubMed]
- Montori VM, Guyatt GH. Intention-to-treat principle. CMAJ 2001;165:1339-41. [PubMed]
- Fergusson D, Aaron SD, Guyatt G, et al. Post-randomisation exclusions: the intention to treat principle and excluding patients from analysis. BMJ 2002;325:652-4. [PubMed]
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med 1998;17:2815-34. [PubMed]
- Nagai K, Tsuchiya R, Mori T, et al. A randomized trial comparing induction chemotherapy followed by surgery with surgery alone for patients with stage IIIA N2 non-small cell lung cancer (JCOG 9209). J Thorac Cardiovasc Surg 2003;125:254-60. [PubMed]
- Vansteenkiste J, Betticher D, Eberhardt W, et al. Randomized controlled trial of resection versus radiotherapy after induction chemotherapy in stage IIIA-N2 non-small cell lung cancer. J Thorac Oncol 2007;2:684-5. [PubMed]
- Betticher DC, Hsu Schmitz SF, Tötsch M, et al. Prognostic factors affecting long-term outcomes in patients with resected stage IIIA pN2 non-small-cell lung cancer: 5-year follow-up of a phase II study. Br J Cancer 2006;94:1099-106. [PubMed]
- Eberhardt W, Wilke H, Stamatis G, et al. Preoperative chemotherapy followed by concurrent chemoradiation therapy based on hyperfractionated accelerated radiotherapy and definitive surgery in locally advanced non-small-cell lung cancer: mature results of a phase II trial. J Clin Oncol 1998;16:622-34. [PubMed]
- Lorent N, De Leyn P, Lievens Y, et al. Long-term survival of surgically staged IIIA-N2 non-small-cell lung cancer treated with surgical combined modality approach: analysis of a 7-year prospective experience. Ann Oncol 2004;15:1645-53. [PubMed]
- Johnson DH, Rusch VW, Turrisi AT. Scalpels, beams, drugs, and dreams: challenges of stage IIIA-N2 non-small-cell lung cancer. J Natl Cancer Inst 2007;99:415-8. [PubMed]
- Katakami N, Tada H, Mitsudomi T, et al. A phase 3 study of induction treatment with concurrent chemoradiotherapy versus chemotherapy before surgery in patients with pathologically confirmed N2 stage IIIA nonsmall cell lung cancer (WJTOG9903). Cancer 2012;118:6126-35. [PubMed]
- Martin J, Ginsberg RJ, Venkatraman ES, et al. Long-term results of combined-modality therapy in resectable non-small-cell lung cancer. J Clin Oncol 2002;20:1989-95. [PubMed]
- Darling GE, Li F, Patsios D, et al. Neoadjuvant chemoradiation and surgery improves survival outcomes compared with definitive chemoradiation in the treatment of stage IIIA N2 non-small-cell lung cancer. Eur J Cardiothorac Surg 2015. [Epub ahead of print]. [PubMed]
- Higgins K, Chino JP, Marks LB, et al. Preoperative chemotherapy versus preoperative chemoradiotherapy for stage III (N2) non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 2009;75:1462-7. [PubMed]
- Shah AA, Berry MF, Tzao C, et al. Induction chemoradiation is not superior to induction chemotherapy alone in stage IIIA lung cancer. Ann Thorac Surg 2012;93:1807-12. [PubMed]
- Le Péchoux C, Tribodet H, Pignon JP. Surgery (S) and radiotherapy (RT) plus adjuvant chemotherapy (CT) versus surgery and radiotherapy in non-small cell lung cancer (NSCLC): ameta-analysis using individual patient data (IPD) fromrandomised clinical trials (RCTs). J Clin Oncol 2007;25:abstr 7521.
- Burdett S, Stewart L. PORT Meta-analysis Group. Postoperative radiotherapy in non-small-cell lung cancer: update of an individual patient data meta-analysis. Lung Cancer 2005;47:81-3. [PubMed]